How to plan and execute a pilot run to validate tooling, assembly processes, and Quality Control before committing to mass production.
Translating prototype performance into repeatable production requires a methodical pilot production run that focuses on validating the manufacturing process rather than the product design. A properly executed pilot production run surfaces tooling weaknesses, assembly bottlenecks, and quality-control gaps under real cycle times and operator conditions, reducing the likelihood of costly rework when volume starts.
You will find in this article tips to define pilot objectives, selecting pilot quantity and production lines, and preparing operator training, together with a metrics-driven methodology for measuring First Pass Yield, cycle time, scrap rate and process capability. It also lays out pragmatic tests for validating tooling, jigs, fixtures and die life under production loads, plus structured steps to finalize work instructions, quality control plans, traceability and corrective actions based on pilot feedback.
Key Takeaways

- Confirm manufacturing capability before committing to volume
- Define pilot size, production line, footprint and training
- Measure FPY, takt time, cycle time, scrap and capability
- Stress test molds, fixtures and tooling for wear
- Lock down work instructions, inspection plans, traceability links
- Use formal Go/No‑Go gates and regulatory release checklists
- be familiar with the PPAP and the R@R concepts
Validate Manufacturing Processes Not Product Design
Pilot objectives must target manufacturing process validation, not product concept checks.
Define measurable outcomes for equipment setup, operator procedure adherence, and inspection gating. Use the pilot run to validate tooling, assembly processes, and quality control before committing to mass production.
Set numerical targets up front, such as for example:
- aim for process capability Cpk ≥ 1.33 for critical dimensions
- reduce defects toward Six Sigma guidance of 3.4 DPMO where feasible.
- specify a target initial pass yield (IPY) such as ≥95% for noncritical assemblies.
- Include acceptable scrap rates and cycle time windows tied to takt time.
Takt Time definition: in lean production, Takt Time is the calculated pace at which a product must be completed to satisfy customer demand. It essentially acts as the “heartbeat” of the production process, aligning manufacturing speed with the rate of customer orders. Takt time is determined by the simple formula: \(\text{Takt Time} = \frac{\text{Total Available Production Time}}{\text{Total Customer Demand for that Period}}\). The primary goal of establishing a takt time is to perfectly match production output with customer requirements, thereby minimizing waste through overproduction or underproduction and ensuring a smooth, continuous workflow. This key lean manufacturing metric is not a measure of how long it takes to produce a single unit (that’s cycle time), but rather the rhythm that the production system must maintain to meet its commitments.

Typical process objectives:
- Confirm machine repeatability under production cadence.
- Validate assembly sequence and torque/force windows.
- Prove inspection repeatability and throughput.
Each bullet shall become a discrete test with pass/fail criteria and measurement method.
Use established sampling and acceptance schemes such as ANSI/ASQ Z1.4 for lot sampling and classify defects by critical, major, minor severity. For critical defects set AQL = 0; for major items consider AQL 0.65–1.5 depending on risk. Capture run-length data to support Weibull or life estimates for tooling and fixture wear.
Weibull distribution in manufacturing validation: the Weibull distribution is a continuous probability distribution that is widely used in reliability engineering to model the time until failure of a component or system. Its strength lies in its flexibility, which is defined by its key parameters:
- Shape parameter (β or k): this is the most crucial parameter as it indicates the nature of the failure rate over time.
- β < 1: suggests a decreasing failure rate, often indicative of “infant mortality” where early failures are common due to manufacturing defects or initial issues.
- β = 1: indicates a constant failure rate, characteristic of random failures during the useful life of a product.
- β > 1: points to an increasing failure rate, signaling wear-out failures as the product ages.
- Scale parameter (η or λ): also known as the characteristic life, represents the time at which 63.2% of the population will have failed. It essentially stretches or compresses the distribution along the time axis.
- Location parameter (γ): this optional third parameter represents a failure-free period. If it’s greater than zero, it indicates a period of time during which no failures are expected to occur.
For more details, see our article specifically on this topic:
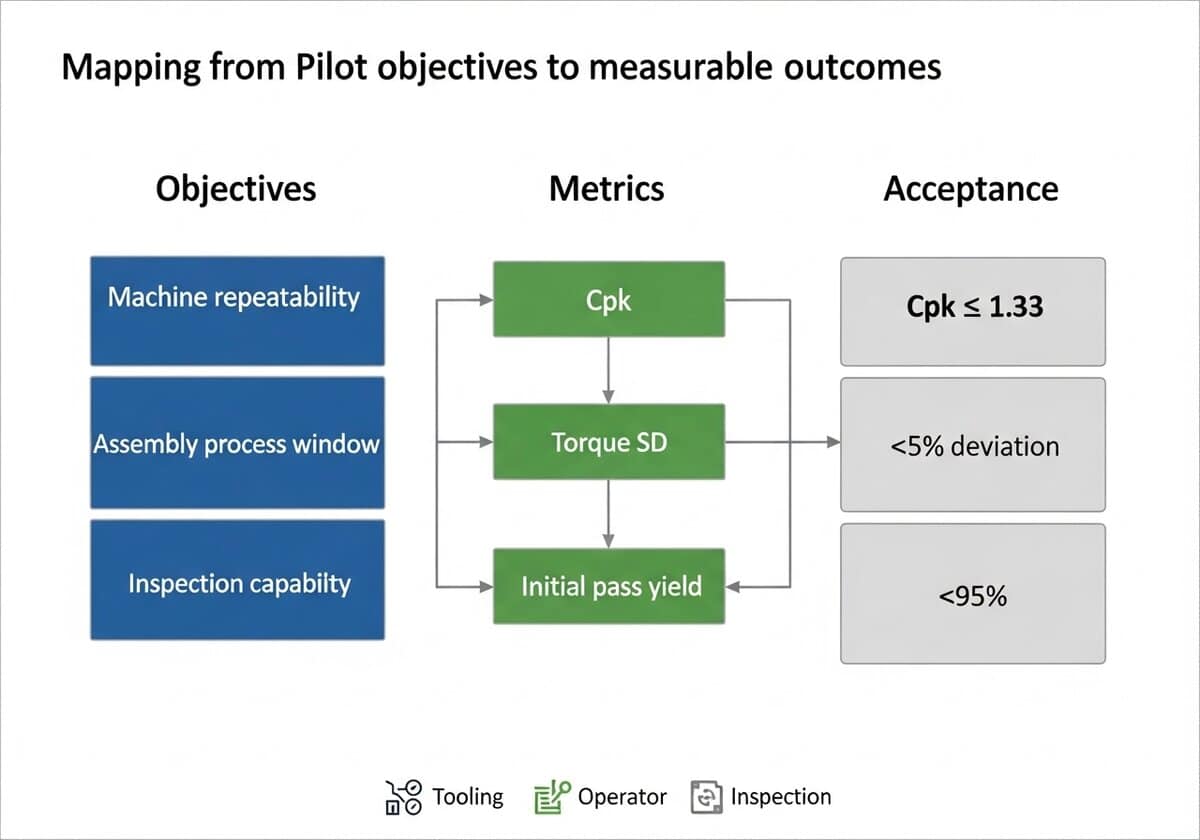
Collect a focused dataset during the trial and map it to decision metrics. The table below summarizes typical pairings.
| Process | Metric | Acceptance |
|---|---|---|
| Injection molding | Dimensional Cpk | ≥1.33 |
| Assembly torque | Torque deviation (SD) | ≤5% of setpoint |
| Inspection | Initial pass yield | ≥95% |
Document objectives, measurement plans, and exit criteria in a pilot protocol signed by both manufacturing and quality together. Include traceability requirements and required data fields for each part number collected.
Tip: require a minimum run length that produces at least 30 independent samples per critical characteristic to support basic capability analysis.
Tip: check with your company rules and domain authority if validation samples shall be kept and for how long.
Planning the Pilot Run

Define the pilot batch count based on validation goals and downstream constraints; common industry practice sets pilot batches between 100–1,000 units to exercise tooling and logistics under production-like cadence. Select quantity to produce statistically meaningful failure modes while limiting scrap and inventory cost.
Choose the production line using clear criteria: equipment match, takt time capability, and operator skill availability. Use an ordered checklist to make the decision reproducible:
- Match core equipment and cycle time
- Confirm material flow and fixtures
- Validate inspection points and traceability
Compare dedicated pilot cell versus using the target production line to decide layout and resource allocation.
| Option | Pros | Cons |
|---|---|---|
| Dedicated pilot cell | Controlled variables, easy observation | Non‑identical equipment |
| Target production line | True process conditions | Disrupts volume output |
Use the table to brief stakeholders and capture trade offs.
Design the shop layout to preserve material flow, ergonomics, and measurement points; place SPC gates where defects first appear. For medical device regulatory compliance, process validation guidance commonly expects at least three consecutive successful batches during performance qualification. Train operators on takt time, defect recognition, and corrective action initiation before the pilot run.
Implement a competency checklist, hands‑on assessments, and live coaching during initial shifts.
Tip:require operators to complete a timed assembly trial and a quality audit score...
You have read 32% of the article. The rest is for our community. Already a member? Log in
(and also to protect our original content from scraping bots)
Innovation.world community
Login or Register (100% free)
View the rest of this article and all members-only content and tools.
Only real engineers, manufacturers, designers, marketers professionals.
No bot, no hater, no spammer.
Frequently Asked Questions
What should pilot run objectives concentrate on when moving from prototype to production?
How do you choose pilot run quantity, production line, and plant layout?
What operator training is required before starting a pilot run?
Which validation metrics should be tracked during the pilot run?
How should tooling, jigs, fixtures, and dies be validated under pilot conditions?
When and how are work instructions, quality plans, and traceability finalized from pilot feedback?
What are formal go/no-go criteria for moving to mass production?
What industry-specific release requirements apply for consumer electronics, injection molded plastics, medical devices, and automotive parts?
Related Topics
- Supplier qualification and incoming materials control: pre-qualifying suppliers and setting material acceptance criteria
- Statistical process control and control-chart deployment: implementing SPC rules and control charts for live process monitoring
- Environmental and accelerated stress testing on production units: executing thermal, humidity and vibration stress runs during pilot
- Packaging, kitting and labeling validation under production throughput: verifying package integrity, kitting accuracy and label application at speed
- Manufacturing execution system (MES) and data-capture integration: connecting machines and operators to capture traceability and analytics
- Regulatory submissions and audit readiness for pilot data: preparing documentation and evidence from pilot runs for regulatory review
- Maintenance strategy validation and mean time tracking: validating preventive maintenance intervals and capturing downtime causes
- Engineering change control (ECO) and documentation flow testing: exercising ECO approvals, revision control and shop-floor distribution
- Process risk assessment and PFMEA updates: updating PFMEA, control plans and mitigation actions from pilot data
- Workplace ergonomics and safety assessment: observing operator posture, access and EHS controls under realistic pace
- Supply chain and inbound logistics rehearsal: running kitting, buffer stocking and just-in-time deliveries to the pilot line
- Cost of quality analysis and scrap accounting: quantifying rework, scrap costs and inspection labor for ramp economics
- Firmware and software production deployment and rollback procedures: validating secure flashing, version control and rollback steps in production
- Line balancing and bottleneck identification with takt time analysis: measuring takt, balancing stations and locating throughput constraints
- Pilot-run inspection equipment calibration and gauge R&R: calibrating inspection tools and performing gauge repeatability and reproducibility studies
External Links on Pilot Production Run
International Standards
(hover the link to see our description of the content)
Glossary of Terms Used
American National Standards Institute (ANSI): a private non-profit organization that oversees the development of voluntary consensus standards for products, services, processes, and systems in the United States, promoting quality, safety, and interoperability across various industries.
Calculation of Process Capability (Cpk): a statistical measure that evaluates a process's ability to produce output within specified limits, calculated by assessing the distance between the process mean and the nearest specification limit, normalized by the process standard deviation.
Contract Manufacturer (CM): a company that produces goods on behalf of another firm, typically following specific design and quality specifications. This arrangement allows the hiring company to focus on core competencies such as marketing and product development while outsourcing manufacturing processes.
Corrective Action and Preventative Action (CAPA): a systematic approach to identifying, investigating, and addressing nonconformities and potential issues to prevent recurrence and ensure compliance with regulatory standards in quality management systems.
Cost of Quality (CoQ): total costs associated with ensuring products or services meet quality standards, including prevention, appraisal, and failure costs. It encompasses expenses incurred to avoid defects, costs of evaluating quality, and costs resulting from defects in products or services.
Critical Control Points (CCP): specific stages in a process where control can be applied to prevent, eliminate, or reduce food safety hazards to acceptable levels. Identifying these points is essential for effective hazard analysis and critical control management in food production systems.
Defects Per Million Opportunities (DPMO): a measurement used in quality control that quantifies the number of defects in a process per one million opportunities for error, calculated by dividing the number of defects by the total number of opportunities and multiplying by one million.
Design Failure Mode and Effects (DFMEA): a systematic approach for identifying potential design-related failures, analyzing their effects on system performance, and prioritizing risks to enhance product reliability and safety during the design phase.
Device Master Record (DMR): a compilation of documents and specifications that provide the necessary information to produce a medical device, including design specifications, production processes, quality assurance measures, and labeling requirements, ensuring compliance with regulatory standards.
Eight Disciplines Problem Solving (8D): a structured problem-solving methodology used to identify, correct, and eliminate recurring issues, consisting of eight steps: team formation, problem description, containment actions, root cause analysis, corrective actions, implementation, prevention, and recognition of team efforts.
Electromagnetic Compatibility (EMC): the ability of electrical devices to operate without interference from external electromagnetic fields and to not emit levels of electromagnetic energy that cause interference to other devices.
Engineering Change Order (ECO): a document that authorizes modifications to a product's design, specifications, or processes, detailing the changes, reasons, and implementation instructions, ensuring proper tracking and management of alterations throughout the product lifecycle.
Failure Mode and Effects Analysis (FMEA): a systematic method for evaluating potential failure modes within a system, process, or product, assessing their effects on performance, and prioritizing risks to improve reliability and safety through corrective actions.
First Pass Yield (FPY): a manufacturing metric that measures the percentage of products produced correctly without rework or defects during the initial production process, indicating efficiency and quality in operations.
Food and Drug Administration (FDA): a federal agency of the United States Department of Health and Human Services responsible for regulating food safety, pharmaceuticals, medical devices, cosmetics, and tobacco products to ensure public health and safety through scientific evaluation and enforcement of compliance standards.
Installation Qualification (IQ): a documented process to verify that equipment or systems are installed according to specifications, including assessment of utilities, environmental conditions, and compliance with design requirements, ensuring readiness for operational qualification.
International Organization for Standardization (ISO): a non-governmental international body that develops and publishes standards to ensure quality, safety, efficiency, and interoperability across various industries and sectors, facilitating global trade and cooperation. Established in 1947, it comprises national standardization organizations from member countries.
Measurement System Analysis (MSA): a statistical method used to evaluate the accuracy, precision, and reliability of measurement processes and instruments, ensuring that data collected is valid and consistent for decision-making in quality control and process improvement.
Non-Destructive Testing (NDT): a method used to evaluate material properties, integrity, or structure without causing damage, employing techniques such as ultrasonic, radiographic, magnetic particle, and dye penetrant testing to detect flaws or discontinuities.
Operational Qualification (OQ): a validation process that ensures equipment or systems operate according to specified requirements within defined limits, confirming that they perform as intended in their operational environment.
Performance Qualification (PQ): a process that verifies a system or equipment operates according to specified requirements under real-world conditions, ensuring it consistently performs its intended function within predetermined limits.
Process Capability Index (Cpk): a statistical measure that quantifies how well a process can produce output within specified limits, indicating the relationship between the process mean and the nearest specification limit, adjusted for process variability.
Process Failure Mode and Effects Analysis (PFMEA): a systematic method for identifying and evaluating potential failures in a manufacturing or business process, assessing their effects on outcomes, and prioritizing actions to mitigate risks.
Production Part Approval Process (PPAP): a standardized procedure used in manufacturing to ensure that suppliers meet quality requirements before mass production, involving documentation and validation of design specifications, process capabilities, and production samples to confirm compliance with customer expectations.
Project Management Office (PMO): a centralized entity within an organization that standardizes project management practices, provides governance, supports project execution, and facilitates resource allocation to ensure alignment with strategic objectives and improve project outcomes.
Repeatability and Reproducibility (R&R): a measurement system's ability to produce consistent results under the same conditions (repeatability) and across different conditions or operators (reproducibility), often evaluated through statistical methods to assess variability and reliability in data collection processes.
Standard Operating Procedure (SOP): a set of step-by-step instructions created to help workers carry out routine operations consistently and efficiently, ensuring compliance with regulations and quality standards.
Statistical Process Control (SPC): a method of quality control that employs statistical techniques to monitor and control a process, ensuring it operates at its full potential by identifying variations and maintaining consistent output within specified limits.
Takt Time: the maximum allowable time to produce a product to meet customer demand, calculated by dividing available production time by required output. It helps synchronize production pace with demand, ensuring efficient workflow and resource allocation.






















Related Posts
Latest Publications & Patents on Covalent Organic Frameworks (COFs)
Latest Publications & Patents on Aerogels and Aerographene
Latest Publications & Patents on High‑Entropy Oxides (HEOs)
Latest Publications & Patents on MXenes
Latest Publications & Patents on Quantum Dots
Latest Publications & Patents on Perovskites