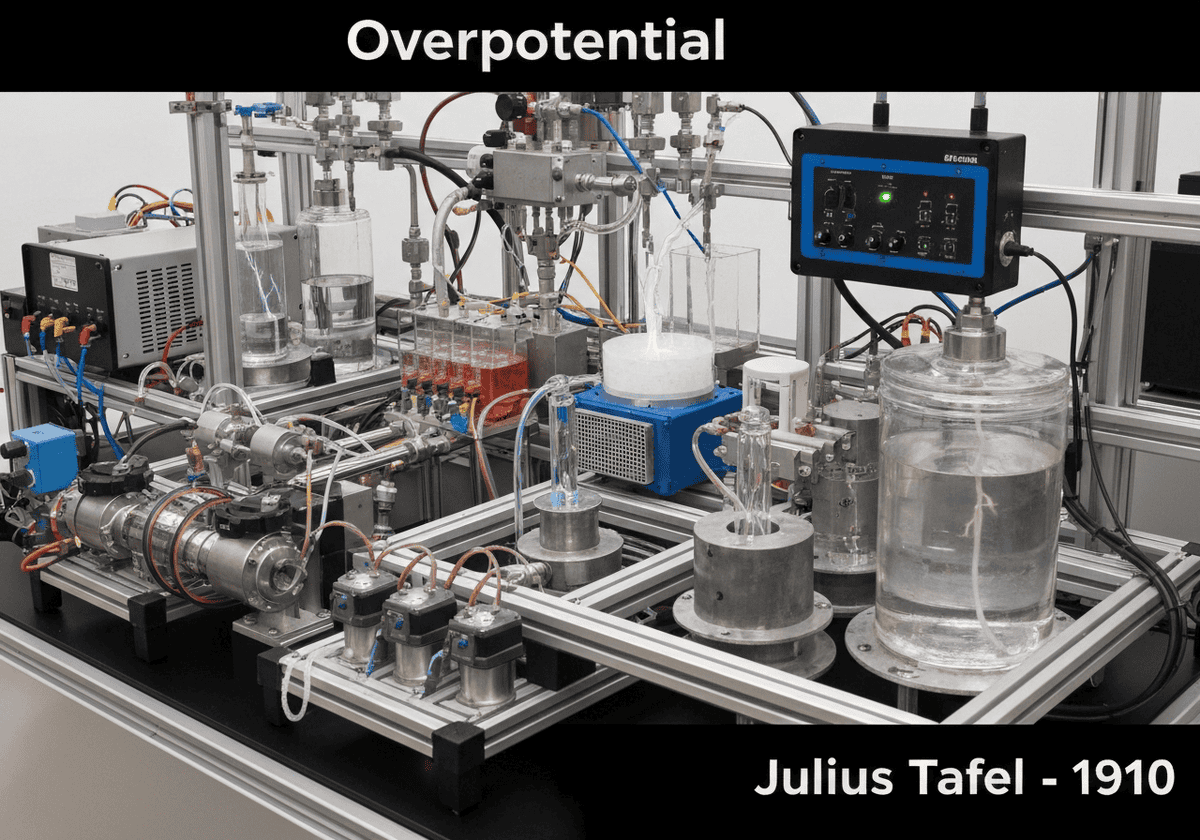
Overpotential is the potential difference (voltage) between a half-reaction’s thermodynamically determined reduction potential and the potential at which the redox event is experimentally observed. It represents the extra energy required to overcome activation barriers for the electrode reaction to proceed at a significant rate. It is a key factor in the energy efficiency of all electrolytic processes.