As global plastic waste reaches staggering levels—an estimated 380 million tons produced annually, with only 9% recycled—the urgency for effective recycling solutions has never been greater. Chemical recycling emerges as a transformative approach, distinct from conventional mechanical methods, offering the potential to recover valuable feedstock from a variety of plastics. This article will provide a comprehensive overview of major chemical recycling technologies, including pyrolysis and gasification, and assess their feedstock requirements for different plastic types. We will evaluate the output products such as monomers and fuels, discuss the current technological readiness levels and scalability of these processes, and analyze their environmental implications and economic feasibility.
Key Takeaways
- Chemical recycling differs from mechanical processes significantly.
- Pyrolysis may convert plastics into fuel and other products.
- Gasification transforms plastics into syngas for energy.
- Feedstock requirements vary based on plastic types processed.
- Output products include monomers, naphtha, and fuels.
- Environmental impact and economic factors influence viability.
Overview of Chemical Recycling and Its Distinction from Mechanical Recycling

Chemical recycling is a transformative approach that involves breaking down plastics at the molecular level to regenerate raw materials suitable for various applications. Unlike
mechanical recycling, which physically processes plastics into smaller pieces without altering their chemical structure, chemical recycling aims to decompose polymers, converting them back into monomers or other chemical building blocks. This process allows for the production of high-quality recycled materials that can be reused to manufacture new products with properties similar to virgin materials.
As an example, a study indicated that chemical recycling could potentially reclaim over 90% of plastics into usable forms, addressing quality concerns often associated with mechanically recycled materials
In contrast, mechanical recycling often suffers from limitations due to contamination, complexity in the feedstock composition, and degradation of material properties upon repeated recycling. For instance, mechanical processes can lead to the loss of some of the physical characteristics of plastics, usually resulting in lower-value applications. This can be quantified by a significant drop in tensile strength, sometimes exceeding 50% for certain polymers after just two cycles of mechanical recycling.
Commonly, chemical recycling can be bifurcated into two main methods:
- depolymerization, which focuses on returning plastics to their monomer states
- pyrolysis, which converts them into fuels and chemicals. Each method has its respective suitability depending on the type of plastic being processed.
For instance, PET (polyethylene terephthalate), commonly used in beverage bottles, can be effectively depolymerized to reclaim its constituent monomers, while polyolefins like polypropylene can be more efficiently processed through pyrolysis.
Despite its promise, the implementation of chemical recycling faces certain challenges, including technological readiness and regulatory hurdles. Several ongoing pilot projects across Europe and North America have reported yields of around 80-90% for specific plastics, showcasing potential feasibility. As technologies evolve, the clear differentiation between chemical and mechanical recycling processes will play a significant role in determining how effective our waste management and recycling systems can be.
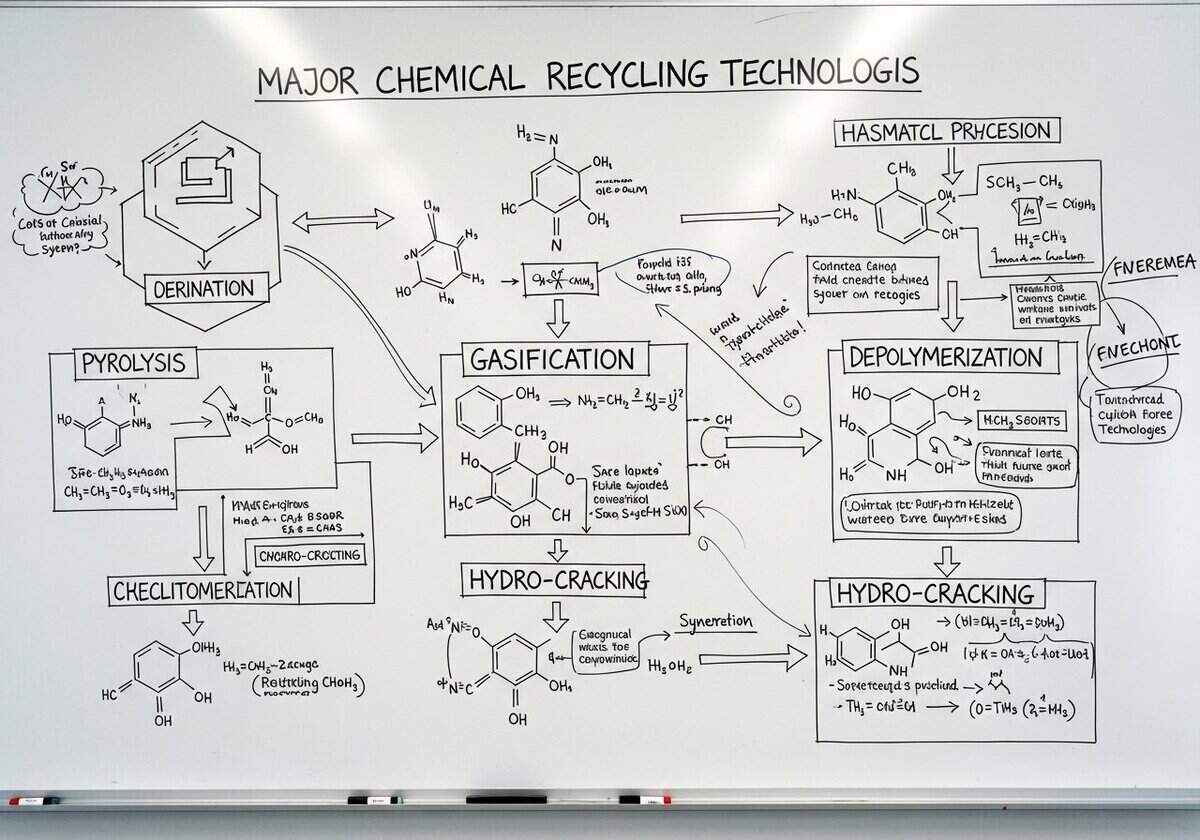
Major Chemical Recycling Technologies: Pyrolysis, Gasification, Depolymerization, and Hydro-cracking

Pyrolysis process: it involves the thermal decomposition of organic materials in the absence of oxygen, converting waste plastics into valuable hydrocarbons. The typical operating temperature ranges from 300°C to 900°C, depending on the type of feedstock and desired end products. Pyrolytic oil can be used as an alternative fuel or upgraded to produce diesel and raw materials for chemical synthesis. A notable example is the conversion of polystyrene into styrene monomer, which has applications in the production of various plastics and resins. Production metrics suggest that pyrolysis can achieve an efficiency rate of up to 80%, recovering significant amounts of energy from waste plastics.
Gasification: it operates on the principle of converting carbonaceous materials into syngas (a mixture of carbon monoxide, hydrogen, and some carbon dioxide) by reacting the materials at high temperatures (around 700°C to 1,600°C) with a controlled amount of oxygen and/or steam. The syngas produced can serve as a fuel source for electricity generation or as a precursor for chemicals like methanol and ammonia. An established facility in Germany, operated by BASF, efficiently gasifies mixed plastic waste with a reported energy recovery of about 60% of the original energy content.

Depolymerization: it requires specific catalysts and conditions to cleave polymer chains into monomers or oligomers. This method is selective for certain types of plastics, such as PET and polyolefins. Recent advancements have improved yields and reduced reaction times, making depolymerization a promising method for reclaiming high-quality raw materials. The Massachusetts Institute of Technology (MIT) has successfully developed a novel catalyst that can increase the depolymerization rate for PET, significantly enhancing recovery efficiency.
Hydro-cracking: it involves the use of hydrogen and specific catalysts to convert larger hydrocarbons into smaller, more valuable ones, typically under high pressure and moderate temperatures (around 300°C to 400°C). Commonly, this method is applied to heavy oils and can also be harnessed for processing plastic waste into usable fuels. For instance, several refinery operations in South Korea effectively utilize hydro-cracking techniques, achieving yields of over 85% in liquid fuels from plastic residues. This significantly reduces landfill reliance while converting waste into economically viable products.
| Technology | Temperature (°C) | End Products | Efficiency (%) |
|---|---|---|---|
| Pyrolysis | 300-900 | Oils, waxes | 80 |
| Gasification | 700-1600 | Syngas | 60 |
| Depolymerization | Varies | Monomers | Up to 95 |
| Hydro-cracking | 300-400 | Liquid fuels | 85 |
Feedstock Requirements and Suitability for Different Plastic Types
The selection of feedstock for chemical recycling processes is dependent on the type of plastic being processed. Different plastics, categorized by their resin identification codes, possess distinct characteristics that affect their suitability for various recycling methods. For example, polyethylene terephthalate (PET), commonly used in beverage bottles, is more amenable to processes like depolymerization, yielding high-quality virgin-like materials. In contrast, polyolefins such as polyethylene (PE) and polypropylene (PP) can be effectively recycled through pyrolysis, converting them into crude oil-like substances.
Polyethylene, with its varying densities (LDPE and HDPE), presents diverse challenges. While LDPE has a lower melting point, making it less stable for thermal processes, HDPE exhibits higher strength and can sustain higher temperatures, enhancing its viability for gasification. The optimal feedstock requirements focus on particle size and impurity levels; feedstock must be sorted to remove contaminants, ensuring high conversion efficiency while minimizing feedstock degradation during processing.
Tip: for optimal results in pyrolysis, maintaining a consistent feedstock composition with a high proportion of thermoplastics improves oil yield and quality. Aim for contamination levels below 5% for better efficiency.
You have read 42% of the article. The rest is for our community. Already a member? Log in
(and also to protect our original content from scraping bots)
Innovation.world community
Login or Register (100% free)
View the rest of this article and all members-only content and tools.
Only real engineers, manufacturers, designers, marketers professionals.
No bot, no hater, no spammer.
Frequently Asked Questions
What is chemical recycling and how does it differ from mechanical recycling?
Chemical recycling involves breaking down plastic waste into its chemical building blocks or raw materials through various processes, allowing for the recycling of a wider range of plastics beyond what mechanical recycling can handle. Mechanical recycling typically involves the physical processing of plastics into flakes or pellets, which may result in degradation of material quality over time.
What are the major chemical recycling technologies?
Key chemical recycling technologies include pyrolysis, gasification, depolymerization (also known as solvolysis), and hydro-cracking. Each technology uses different methods to convert waste plastics back into valuable products, such as fuels or raw materials for new plastic production.
What feedstock requirements do chemical recycling processes have?
The suitability of feedstock varies significantly between chemical recycling methods, with each technology best suited for specific types of plastics. For instance, pyrolysis is effective for mixed plastic waste, while depolymerization excels with polyesters or polyamides, requiring better sorting.
What products can be generated from chemical recycling processes?
Chemical recycling can produce a range of output products, including monomers, naphtha, syngas, and alternative fuels. These products can be repurposed within the chemical industry, either for the production of new plastics or to create energy sources.
How does chemical recycling fit into plastics waste management and the circular economy?
Chemical recycling plays a significant role in plastics waste management by enabling the recovery and reuse of plastics that would otherwise be discarded. This supports circular economy initiatives, where materials are continually reused, reducing landfill dependency and optimizing resource utilization.
Related Topics
- Emerging Chemical Recycling Technologies: new and innovative methods being developed in the field of chemical recycling.
- Feedstock Quality Assessment: evaluation criteria for determining the suitability of feedstock in recycling processes.
- Life Cycle Assessment (LCA): an analysis method to assess the environmental impacts of chemical recycling throughout its life cycle.
- Policy and Regulatory Frameworks: guidelines and laws influencing the practice and development of chemical recycling technologies.
- Community Engagement in Recycling Programs: the role of public awareness and participation in enhancing recycling initiatives.
- Infrastructure Development for Recycling Facilities: the necessary physical resources and systems required for supporting chemical recycling operations.
- Recyclability Evaluation Standards: metrics and criteria used to determine the recyclability of various materials.
- End-of-Life Solutions for Plastic Products: strategies addressing the disposal and recycling of plastics at their lifecycle’s end.
- Consumer Perception of Recycling: insights into how public attitudes affect recycling behaviors and program success.
- Circular Supply Chains: systems designed to maintain materials within the economy for as long as possible through recycling.
External Links on Plastics Recycling
International Standards
(hover the link to see our description of the content)
Glossary of Terms Used
Life Cycle Assessment (LCA): a systematic analysis of the environmental impacts associated with all stages of a product's life, from raw material extraction through production, use, and disposal, aimed at identifying opportunities for improvement and informing decision-making.
Positron Emission Tomography (PET): a medical imaging technique that detects gamma rays emitted by positron annihilation, used to visualize metabolic processes in tissues, often employing radiotracers to assess conditions such as cancer, neurological disorders, and cardiovascular diseases.
Technological Readiness Levels (TRL): a scale used to assess the maturity of a technology, ranging from basic research and development to full deployment, typically categorized from 1 (concept) to 9 (operational use), facilitating evaluation and decision-making in technology development processes.

























Has anyone considered the energy consumption for these chemical recycling processes compared to mechanical recycling?
the energy cost for these chemical recycling processes wasnt too much mentioned in the article ..
What about the energy consumption of these chemical processes? That a major environmental concern too
Related Posts
Latest Publications & Patents on Perovskites
Latest Publications & Patents on Graphene
45+ Science Tricks for Games and Marketing: Data-Driven and Statistical Tricks
Use or Abuse 25 Cognitive Biases in Product Design and Manufacturing
Revised NIOSH Lifting Equation in Bench Ergonomics
Dark Web vs Darknet vs Deep Web: 101 & More