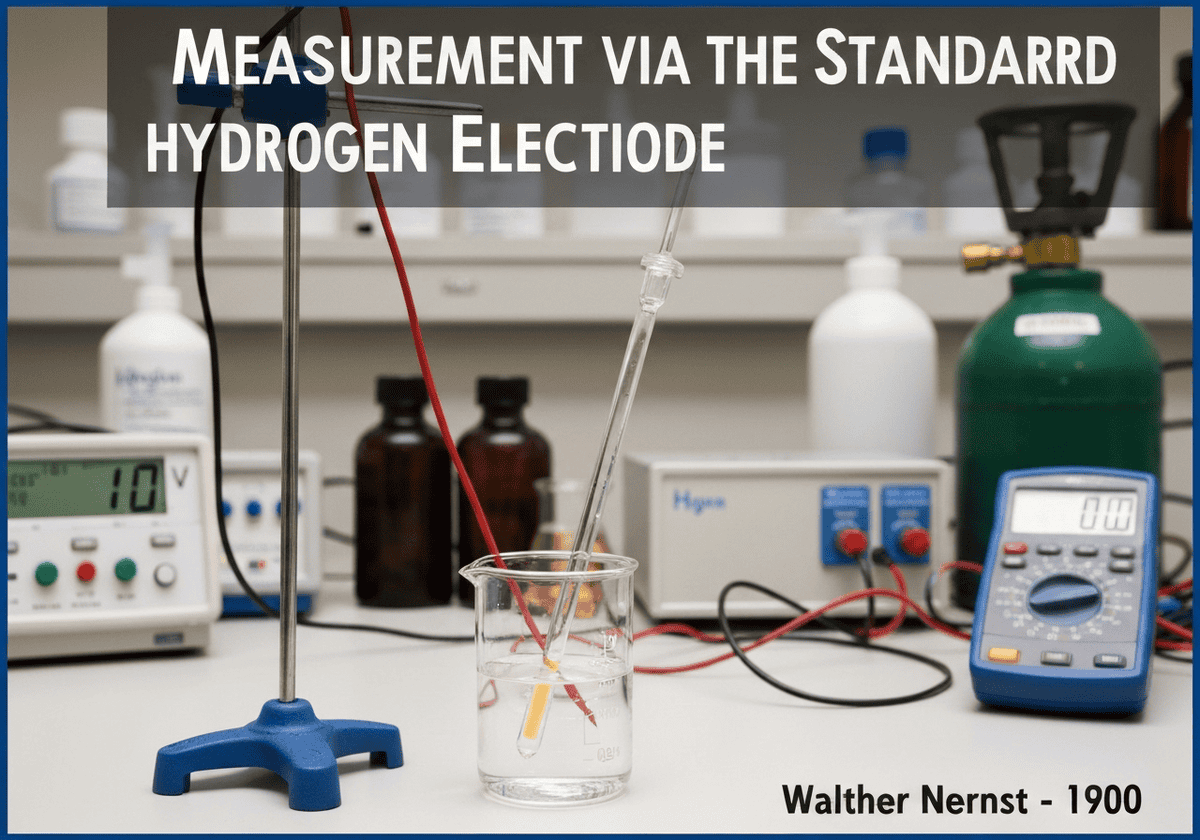
The absolute electrochemical potential of a single electrode cannot be measured. Instead, potentials are measured relative to a universally accepted reference. The Standard Hydrogen Electrode (SHE) is the primary reference, assigned a potential of exactly 0 volts under standard conditions (1 M \(H^+\) concentration, 1 bar \(H_2\) gas, 298 K). All other standard electrode potentials are reported relative to this zero point.