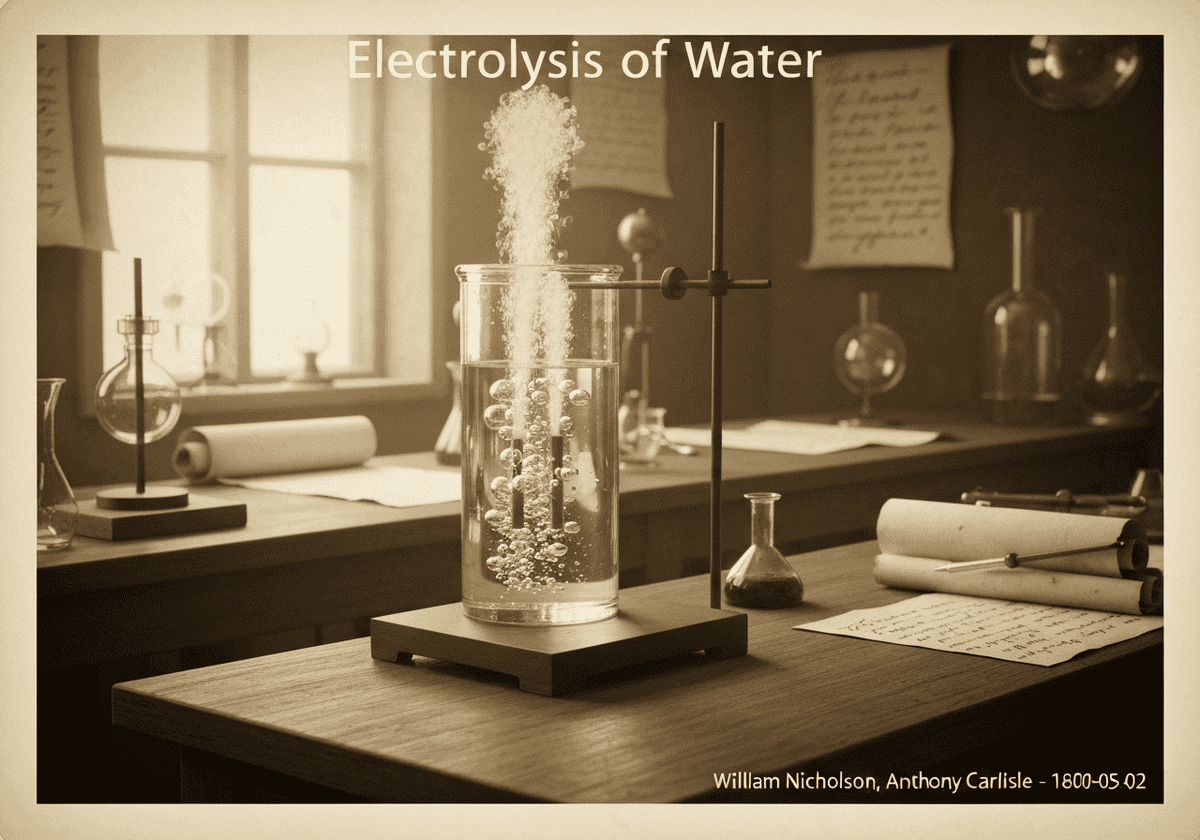
The electrolysis of water is the decomposition of water (H₂O) into its constituent elements, oxygen (O₂) and hydrogen (H₂), by passing an electric current through it. At the cathode, two water molecules are reduced to form hydrogen gas and hydroxide ions. At the anode, two water molecules are oxidized to form oxygen gas, protons, and electrons.