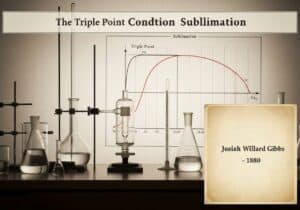
Le Chatelier’s principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium moves to counteract the change. This principle, also known as the equilibrium law, predicts the qualitative effect of a change in concentration, temperature, or pressure on a chemical system at equilibrium. It guides understanding of how reversible reactions respond to external stresses.