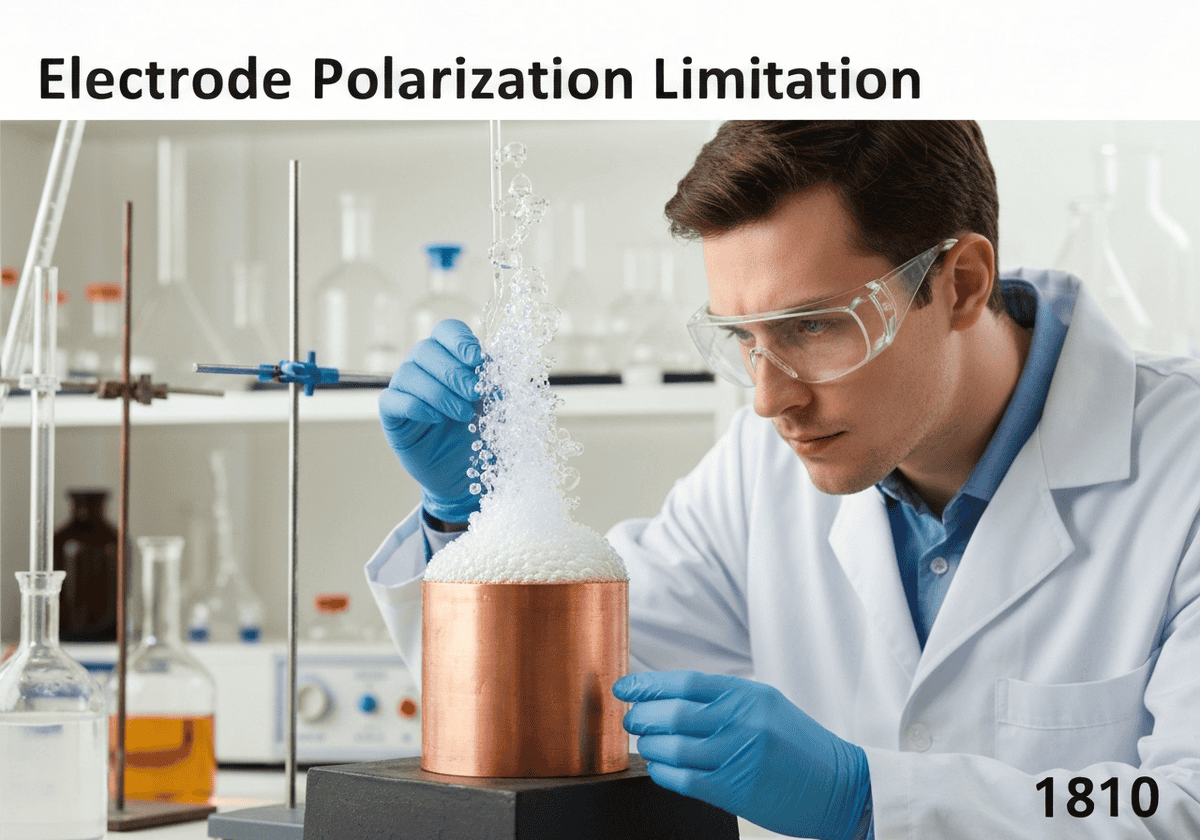
A primary flaw of the Voltaic pile is electrode polarization. During operation, hydrogen gas produced at the copper cathode forms an insulating layer of bubbles on its surface. This layer increases the battery’s internal resistance and reduces the surface area available for reaction. As a result, the voltage and current output of the pile drop significantly shortly after it begins to be used.