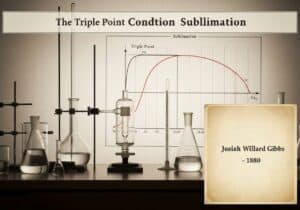
An equation of state for a fluid that modifies the ideal gas law to approximate the behavior of real gases. It introduces two parameters: ‘a’ to account for long-range intermolecular attractive forces (Van der Waals forces) and ‘b’ for the finite volume occupied by the gas molecules. The equation is \((P + \frac{an^2}{V^2})(V – nb) = nRT\).