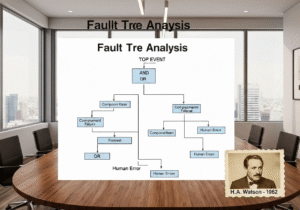
Good Manufacturing Practice (BPF) is a system of regulations and guidelines ensuring that pharmaceutical products are consistently produced and controlled according to quality standards. It covers all aspects of production, from the starting materials, premises, and equipment to the training and personal hygiene of staff. GMP is designed to minimize the risks involved in any pharmaceutical production.