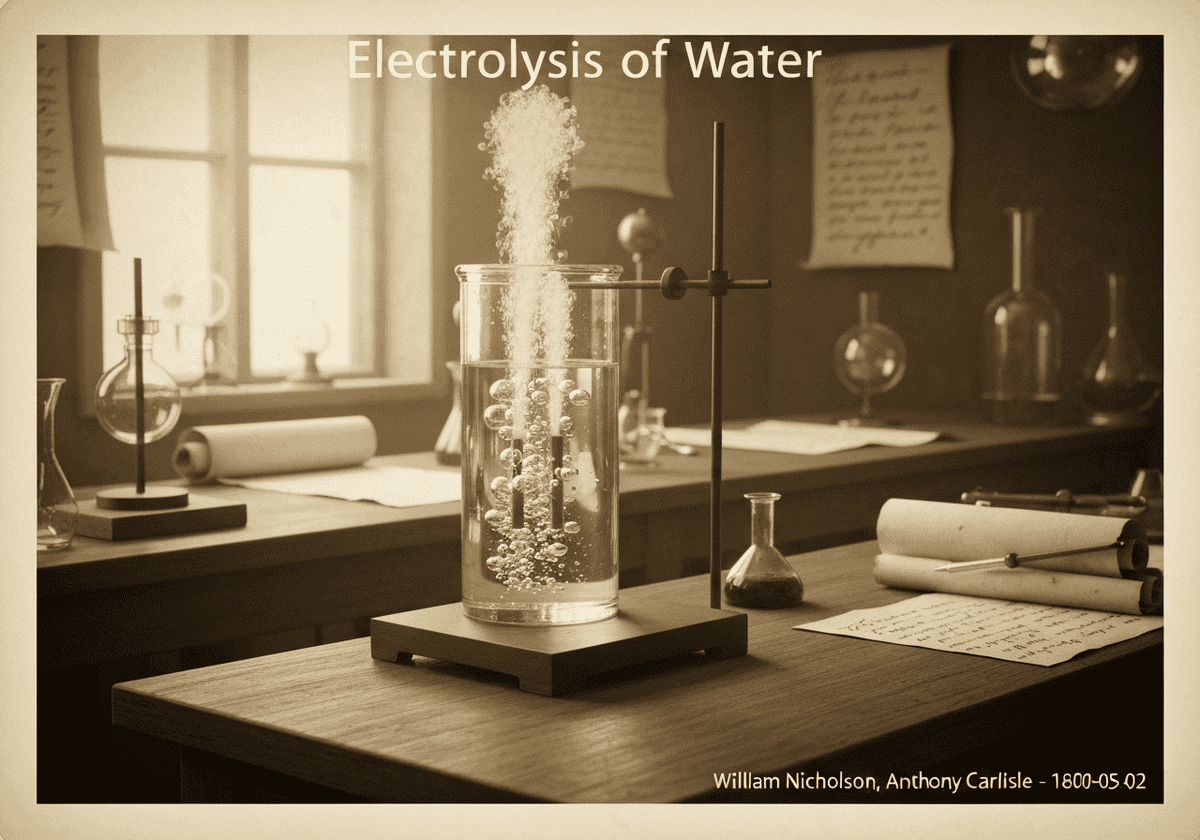
Bei der Wasserelektrolyse wird Wasser (H₂O) durch Durchleiten von elektrischem Strom in seine Bestandteile Sauerstoff (O₂) und Wasserstoff (H₂) zerlegt. An der Kathode werden zwei Wassermoleküle zu Wasserstoffgas und Hydroxidionen reduziert. An der Anode werden zwei Wassermoleküle zu Sauerstoffgas, Protonen und Elektronen oxidiert.