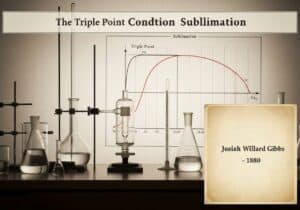
A direct consequence of Avogadro’s law is the concept of molar volume: one mole of any ideal gas at a specified temperature and pressure occupies a fixed volume. At Standard Temperature and Pressure (STP), defined as 273.15 K (0 °C) and 1 atm, this volume is approximately 22.4 liters. This principle simplifies stoichiometry by allowing direct conversion between gas volume and moles.