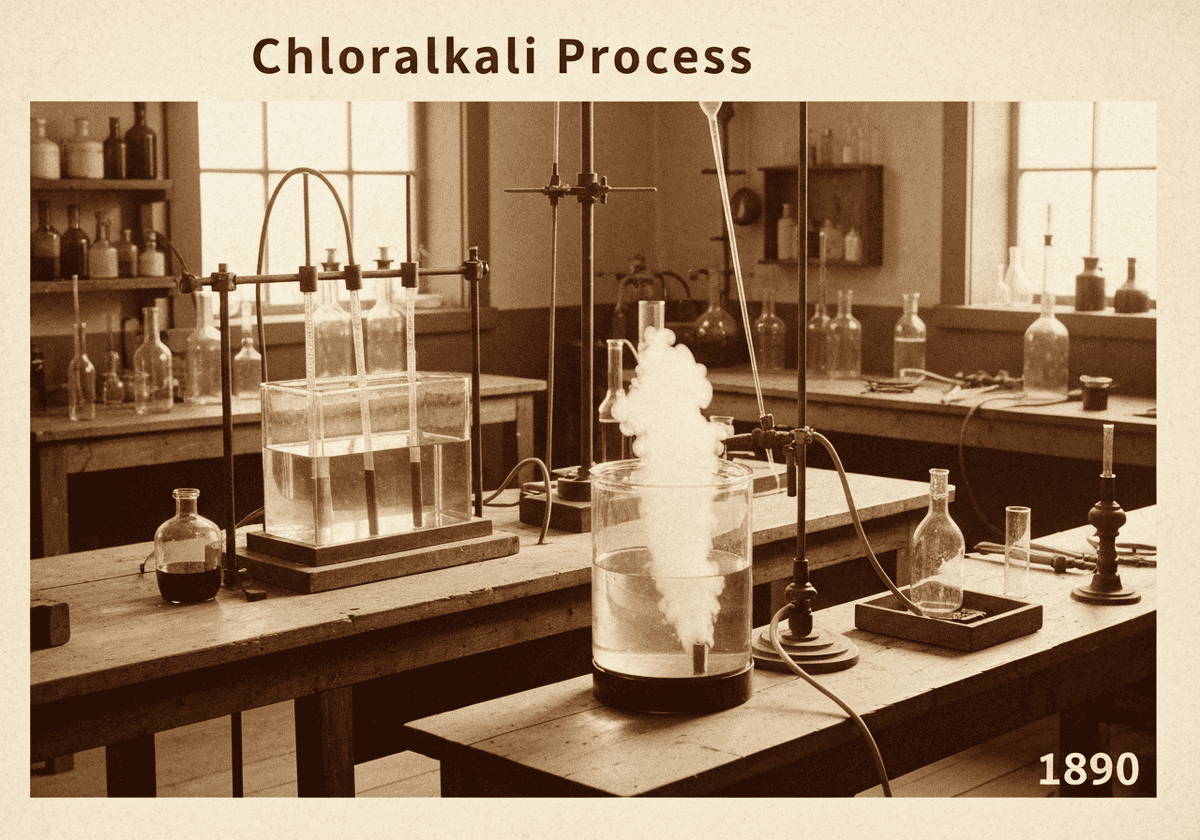
The chloralkali process is an industrial method for the electrolysis of sodium chloride (NaCl) solution, known as brine. It is the primary source for producing chlorine (Cl₂), sodium hydroxide (NaOH), and hydrogen (H₂), which are essential commodity chemicals. Modern methods use a membrane cell to separate the anode and cathode products, ensuring high purity and efficiency.