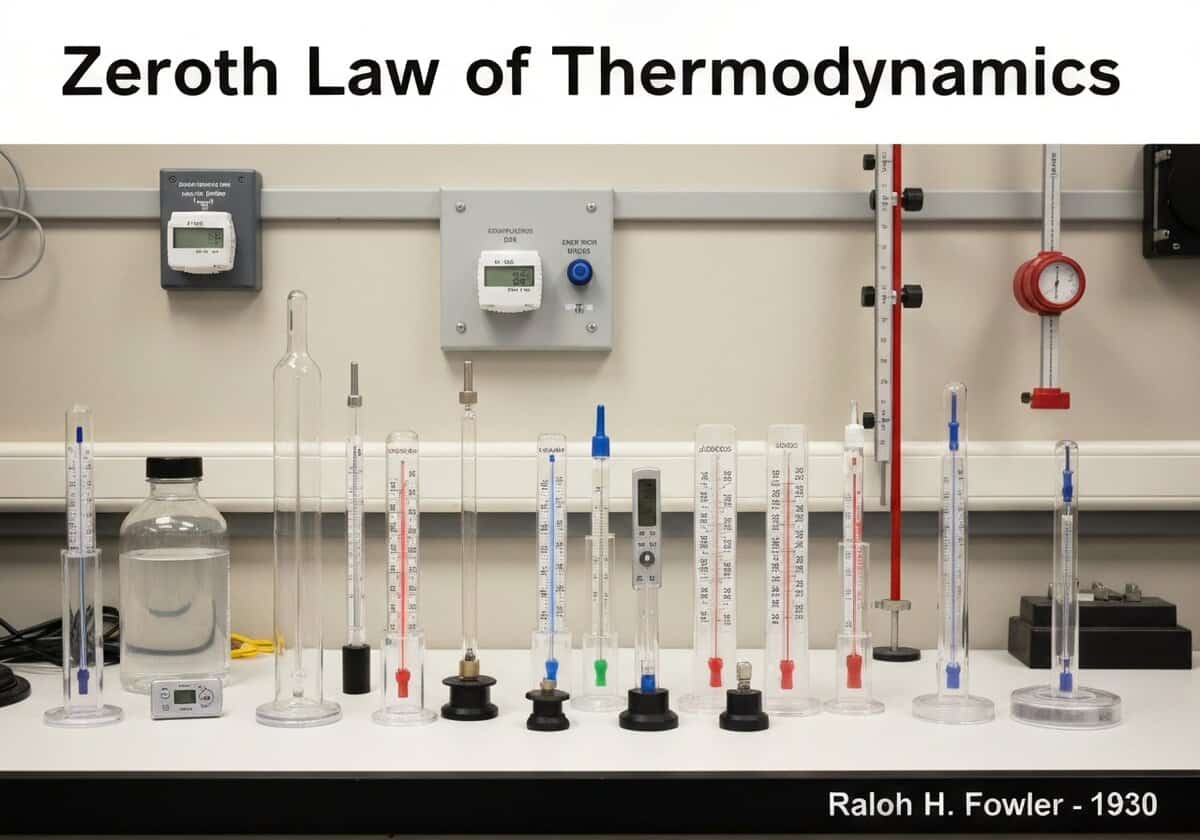
This law establishes the concept of thermal equilibrium. If two systems are each in thermal equilibrium with a third, independent system, they are in thermal equilibrium with each other. This transitive property allows for the formal definition of temperature as a state function and provides a rational basis for constructing thermometers and universal temperature scales.