Insulin is synthesized in pancreatic beta cells as a single-chain precursor called proinsulin. In the endoplasmic reticulum, proinsulin folds and forms its disulfide bonds. It is then transported to the Golgi apparatus and packaged into secretory granules, where proteases (prohormone convertases PC1/3 and PC2, and carboxypeptidase E) cleave it into mature insulin and a connecting peptide, C-peptide.
Proinsulin Processing and Insulin Biosynthesis
- Donald F. Steiner
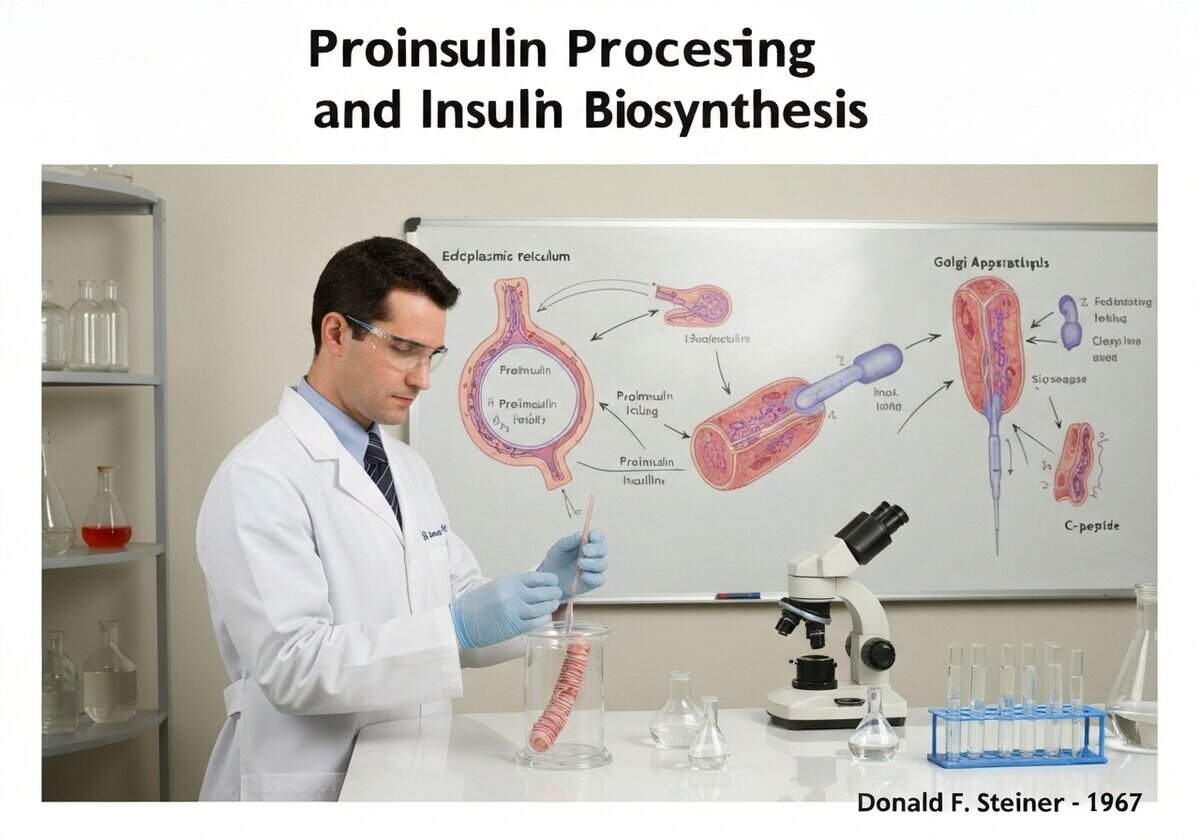
The discovery of proinsulin by Donald F. Steiner in 1967 resolved a key puzzle in insulin biosynthesis. It was known that insulin consisted of two separate polypeptide chains (A and B), but the mechanism for ensuring their coordinated synthesis and correct assembly via disulfide bonds was unclear. Steiner’s research revealed that a single gene codes for a larger, single-chain precursor. This preproinsulin molecule contains a signal peptide at its N-terminus that directs it into the lumen of the endoplasmic reticulum (ER). The signal peptide is quickly cleaved off, leaving proinsulin. Proinsulin consists of the B chain, a connecting C-peptide, and the A chain, in that order (B-C-A). Within the ER, the molecule folds into its proper conformation, guided by chaperones, which brings the cysteine residues into proximity, allowing the three crucial disulfide bonds to form correctly. This folded proinsulin is then transported to the Golgi complex and packaged into immature clathrin-coated secretory vesicles. As these vesicles mature into dense-core secretory granules, the internal environment becomes more acidic, activating specific endoproteases. Prohormone convertase 1/3 (PC1/3) makes the initial cut at the B-chain/C-peptide junction, and prohormone convertase 2 (PC2) cuts at the C-peptide/A-chain junction. Finally, carboxypeptidase E (CPE) trims off basic amino acid residues at the new C-termini, resulting in the final, mature two-chain insulin molecule and the free C-peptide. Both are stored in these granules and co-secreted in equimolar amounts in response to high blood glucose.
Tipo
Disrupción
Utilización
Precursores
- discovery of the protein synthesis pathway (ribosomes, er, golgi)
- determination of insulin’s two-chain structure by sanger
- development of pulse-chase labeling techniques using radioactive amino acids
- identification of proteolytic enzymes (proteases)
Aplicaciones
- measurement of c-peptide levels in blood to assess endogenous insulin production
- understanding of congenital hyperproinsulinemia, a genetic disorder of proinsulin processing
- development of single-chain insulin analogs for improved stability
- research into protein folding and trafficking within the cell
- providing a model system for the biosynthesis of other peptide hormones
Patentes:
Posibles ideas innovadoras
Membresía obligatoria de Professionals (100% free)
Debes ser miembro de Professionals (100% free) para acceder a este contenido.
DISPONIBLE PARA NUEVOS RETOS
Ingeniero Mecánico, Gerente de Proyectos, Ingeniería de Procesos o I+D
Disponible para un nuevo desafío a corto plazo.
Contáctame en LinkedIn
Integración de electrónica de metal y plástico, diseño a coste, GMP, ergonomía, dispositivos y consumibles de volumen medio a alto, fabricación eficiente, industrias reguladas, CE y FDA, CAD, Solidworks, cinturón negro Lean Sigma, ISO 13485 médico
Estamos buscando un nuevo patrocinador
¿Su empresa o institución se dedica a la técnica, la ciencia o la investigación?
> Envíanos un mensaje <
Recibe todos los artículos nuevos
Gratuito, sin spam, correo electrónico no distribuido ni revendido.
o puedes obtener tu membresía completa -gratis- para acceder a todo el contenido restringido >aquí<
Invención, innovación y principios técnicos relacionados











