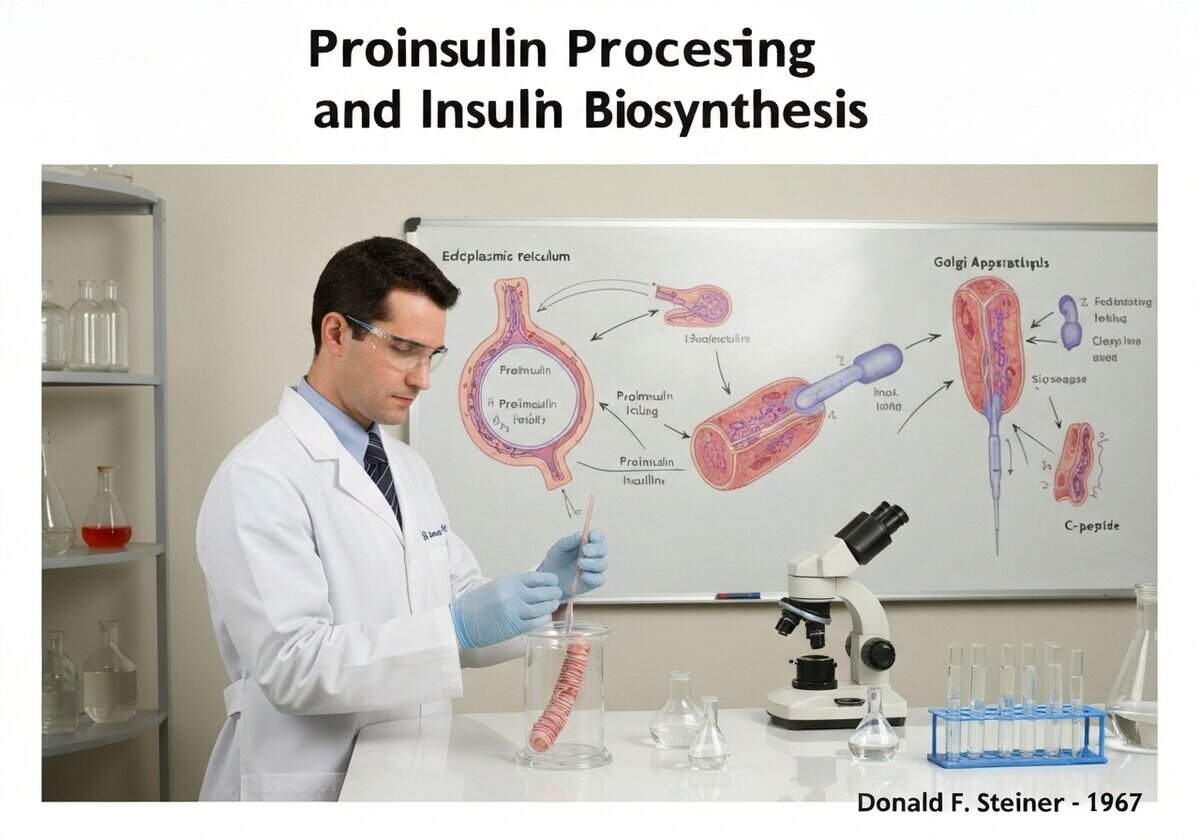
Insulin is synthesized in pancreatic beta cells as a single-chain precursor called proinsulin. In the endoplasmic reticulum, proinsulin folds and forms its disulfide bonds. It is then transported to the Golgi apparatus and packaged into secretory granules, where proteases (prohormone convertases PC1/3 and PC2, and carboxypeptidase E) cleave it into mature insulin and a connecting peptide, C-peptide.
The discovery of proinsulin by Donald F. Steiner in 1967 resolved a key puzzle in insulin biosynthesis. It was known that insulin consisted of two separate polypeptide chains (A and B), but the mechanism for ensuring their coordinated synthesis and correct assembly via disulfide bonds was unclear. Steiner’s research revealed that a single gene codes for a larger, single-chain precursor. This preproinsulin molecule contains a signal peptide at its N-terminus that directs it into the lumen of the endoplasmic reticulum (ER). The signal peptide is quickly cleaved off, leaving proinsulin. Proinsulin consists of the B chain, a connecting C-peptide, and the A chain, in that order (B-C-A). Within the ER, the molecule folds into its proper conformation, guided by chaperones, which brings the cysteine residues into proximity, allowing the three crucial disulfide bonds to form correctly. This folded proinsulin is then transported to the Golgi complex and packaged into immature clathrin-coated secretory vesicles. As these vesicles mature into dense-core secretory granules, the internal environment becomes more acidic, activating specific endoproteases. Prohormone convertase 1/3 (PC1/3) makes the initial cut at the B-chain/C-peptide junction, and prohormone convertase 2 (PC2) cuts at the C-peptide/A-chain junction. Finally, carboxypeptidase E (CPE) trims off basic amino acid residues at the new C-termini, resulting in the final, mature two-chain insulin molecule and the free C-peptide. Both are stored in these granules and co-secreted in equimolar amounts in response to high blood glucose.