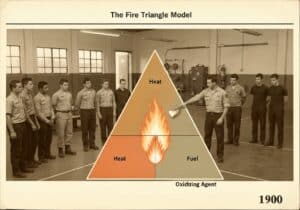
Smothering, or oxygen deprivation, is a method of fire extinguishment that removes the ‘oxidizing agent’ from the fire triangle. This is achieved by covering the fire to prevent air from reaching the fuel. Common methods include using a fire blanket, sand, or deploying an inert gas like carbon dioxide (CO2) or nitrogen.
Extinguishment by smothering works by isolating the fuel from its source of oxygen, thereby breaking the fire triangle. For combustion to occur, the concentration of oxygen in the atmosphere typically needs to be above 16%. Smothering aims to reduce this concentration below the sustainable limit. This can be done physically or chemically. Physical methods involve placing a barrier between the fire and the air. A fire blanket, for example, is a non-flammable sheet used to cover a fire, cutting off the oxygen supply. Similarly, putting a lid on a burning pan achieves the same effect on a smaller scale. Chemical methods involve flooding an area with an inert gas that displaces the oxygen. Carbon dioxide (CO2) is a common agent used in extinguishers and fixed systems. When released, the heavy CO2 gas sinks and blankets the fire, pushing the lighter, oxygen-rich air away. Other inert gases like nitrogen and argon are also used, particularly in systems designed to protect sensitive equipment where the thermal shock from CO2 could cause damage. This 方法 is highly effective in enclosed spaces but less so in open areas where wind can quickly dissipate the smothering agent.