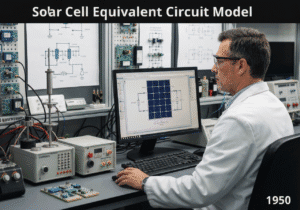
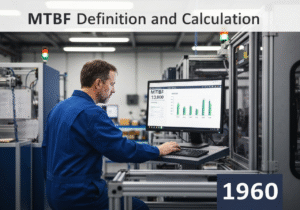
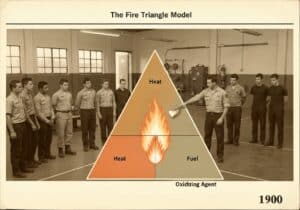
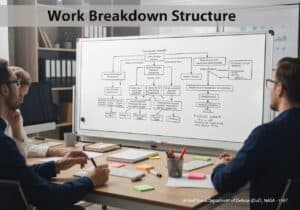
A Pourbaix diagram, also known as a potential/pH diagram, is a termodinamico chart that maps the stable equilibrium phases of an aqueous electrochemical system. It graphically shows the conditions of potential ([latex]E_H[/latex]) and pH under which a metal is immune (thermodynamically stable), passivated (forms a stable protective film), or susceptible to corrosion (forms soluble ions).