A document that describes the systems and processes used to control product and process quality to ensure customer requirements are met.
- Méthodologies : Clients et marketing, Économie, Conception de Produits
Plan de contrôle

Plan de contrôle
- Planification avancée de la qualité des produits (APQP), Carte de contrôle, Mesure corrective, Amélioration des processus, Assurance qualité, Contrôle de qualité, Gestion de la qualité, Système de gestion de la qualité (SMQ), Contrôle statistique des processus (CSP)
Objectif :
Comment il est utilisé :
- Used in manufacturing (often as part of APQP), it is a living document that outlines the measurements, inspections, and checks that will be performed at each stage of the process to ensure output is consistent.
Avantages
- Provides a structured and standardized approach to quality control, ensures all critical characteristics are monitored, and serves as a key reference document for production staff.
Inconvénients
- Can be bureaucratic and time-consuming to create and maintain, requires discipline to follow consistently, and may not be flexible enough to handle unexpected process variations.
Catégories :
- Ingénierie, Lean Sigma, Fabrication, Qualité
Idéal pour :
- Documenting the specific quality control methods and actions used to control a manufacturing process and ensure product quality.
The Control Plan methodology is widely utilized across industries such as automotive, aerospace, electronics, and medical devices, where ensuring product quality is paramount. In the context of Advanced Product Quality Planning (APQP), it is particularly relevant during the design and development phase, as well as during ongoing production, encompassing all stages from initial design validation to final assembly. Key participants in developing and maintaining a Control Plan typically include quality engineers, manufacturing engineers, process managers, and cross-functional team members, who collaborate to ensure that all significant process variables are defined and monitored systematically. The Control Plan is inherently a living document, meaning it must be continuously updated based on process changes, improved technologies, or new customer requirements, thereby facilitating a proactive approach to quality management rather than a reactive one. Typical applications include outlining specific measurement techniques such as Contrôle statistique des processus (SPC), capability studies, and inspection methods that ensure compliance with design specifications. The use of this methodology not only enhances traceability and accountability in manufacturing processes but also serves as a training resource for new employees, reinforcing the organization’s commitment to maintaining high quality standards throughout the production lifecycle. Additionally, the alignment of the Control Plan with other quality management tools, such as Failure Mode and Effects Analysis (FMEA) and Design Reviews, promotes a holistic approach to quality assurance, thus increasing customer satisfaction and reducing product costs associated with defects and rework.
Principales étapes de cette méthodologie
- Identify critical characteristics for the product and process.
- Develop measurement criteria and methods for each characteristic.
- Establish control methods and inspection frequencies.
- Document responsibilities for monitoring and actions taken.
- Integrate feedback loops for continuous improvement.
- Review and update the Control Plan regularly based on process changes.
Conseils de pro
- Regularly update the Control Plan as changes occur in materials, processes, or equipment, ensuring alignment with the latest production standards.
- Integrate real-time data collection and analysis tools within the Control Plan to enhance decision-making and prompt corrective actions during the manufacturing process.
- Include a robust training section within the Control Plan for personnel, emphasizing the importance of adherence to quality checks and continuous improvement procedures.
Lire et comparer plusieurs méthodologies, nous recommandons le
> Référentiel méthodologique étendu <
ainsi que plus de 400 autres méthodologies.
Vos commentaires sur cette méthodologie ou des informations supplémentaires sont les bienvenus sur le site web de la Commission européenne. section des commentaires ci-dessous ↓ , ainsi que toute idée ou lien en rapport avec l'ingénierie.
Contexte historique
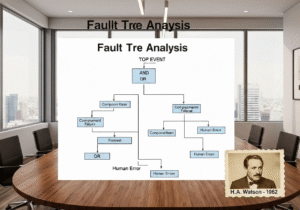
1962
1970
1972
1980
1980
1986
1986
1960
1963
1970
1980
1980
1980
1986
1987
(si la date est inconnue ou n'est pas pertinente, par exemple "mécanique des fluides", une estimation arrondie de son émergence notable est fournie)











Articles Similaires
Questionnaires sur les troubles musculo-squelettiques
Tests à plusieurs variables (MVT)
Analyse de régression multiple
Systèmes de capture de mouvement
Méthode MoSCoW
Test de la médiane de Mood