Sublimation, the direct phase transition from solid to gas, occurs at temperatures and pressures below a substance’s triple point. The triple point is the unique termodinámica state where the solid, liquid, and gas phases can coexist in equilibrium. On a phase diagram, the sublimation curve separates the solid and gas phases, originating at the origin and ending at the triple point.
The Triple Point Condition for Sublimation
- Josiah Willard Gibbs
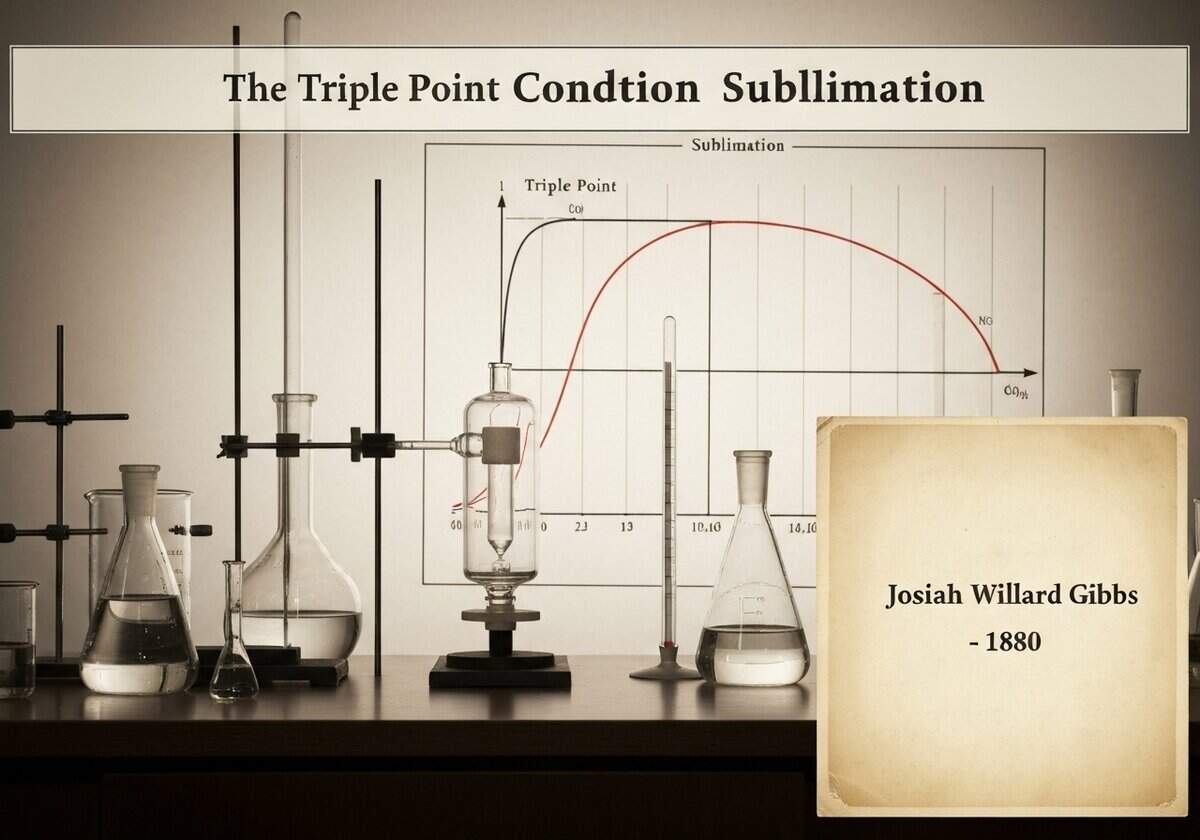
The concept of the triple point is a cornerstone of thermodynamics, formally described by Josiah Willard Gibbs’ phase rule. The phase rule, [latex]F = C – P + 2[/latex], relates the number of degrees of freedom (F) of a system to the number of components (C) and the number of phases (P) in equilibrium. For a pure substance (C=1), if three phases coexist (P=3), the number of degrees of freedom is F = 1 – 3 + 2 = 0. This means the state is invariant; it can only exist at a single, specific combination of temperature and pressure, which is the triple point.
The phase diagram of a substance graphically represents the conditions under which its different phases are stable. It is typically a plot of pressure versus temperature. The diagram is divided into solid, liquid, and gas regions by phase boundaries. The line separating the solid and gas regions is the sublimation curve. Any point on this line represents a state where the solid and gas phases are in equilibrium. If one starts in the solid phase region and lowers the pressure at a constant temperature, crossing this line will induce sublimation. Similarly, heating a solid at a constant pressure that is below the triple point pressure will also cause it to sublime. For water, the triple point is at 0.01 °C (273.16 K) and a pressure of 611.657 Pa. Any pressure below this value will cause ice to sublime directly into water vapor upon heating, bypassing the liquid water phase entirely. This principle is fundamental to processes like freeze-drying, where low pressure is applied to frozen materials to remove water content efficiently without the damaging effects of high heat.
Tipo
Disrupción
Utilización
Precursores
- studies on the states of matter by antoine lavoisier
- development of the kinetic theory of gases by clausius and maxwell
- formulation of the laws of thermodynamics
- early observations of phase changes, such as the boiling of water and melting of ice
Aplicaciones
- freeze-drying (lyophilization) of food and pharmaceuticals
- understanding comet tail formation in the vacuum of space
- frost formation (deposition)
- purification of chemical compounds via vacuum sublimation
- manufacturing of dry ice
Patentes:
Posibles ideas innovadoras
Membresía obligatoria de Professionals (100% free)
Debes ser miembro de Professionals (100% free) para acceder a este contenido.
DISPONIBLE PARA NUEVOS RETOS
Ingeniero Mecánico, Gerente de Proyectos, Ingeniería de Procesos o I+D
Disponible para un nuevo desafío a corto plazo.
Contáctame en LinkedIn
Integración de electrónica de metal y plástico, diseño a coste, GMP, ergonomía, dispositivos y consumibles de volumen medio a alto, fabricación eficiente, industrias reguladas, CE y FDA, CAD, Solidworks, cinturón negro Lean Sigma, ISO 13485 médico
Estamos buscando un nuevo patrocinador
¿Su empresa o institución se dedica a la técnica, la ciencia o la investigación?
> Envíanos un mensaje <
Recibe todos los artículos nuevos
Gratuito, sin spam, correo electrónico no distribuido ni revendido.
o puedes obtener tu membresía completa -gratis- para acceder a todo el contenido restringido >aquí<
Invención, innovación y principios técnicos relacionados














