Sublimation, the direct phase transition from solid to gas, occurs at temperatures and pressures below a substance’s triple point. The triple point is the unique 热力学 state where the solid, liquid, and gas phases can coexist in equilibrium. On a phase diagram, the sublimation curve separates the solid and gas phases, originating at the origin and ending at the triple point.
The Triple Point Condition for Sublimation
- Josiah Willard Gibbs
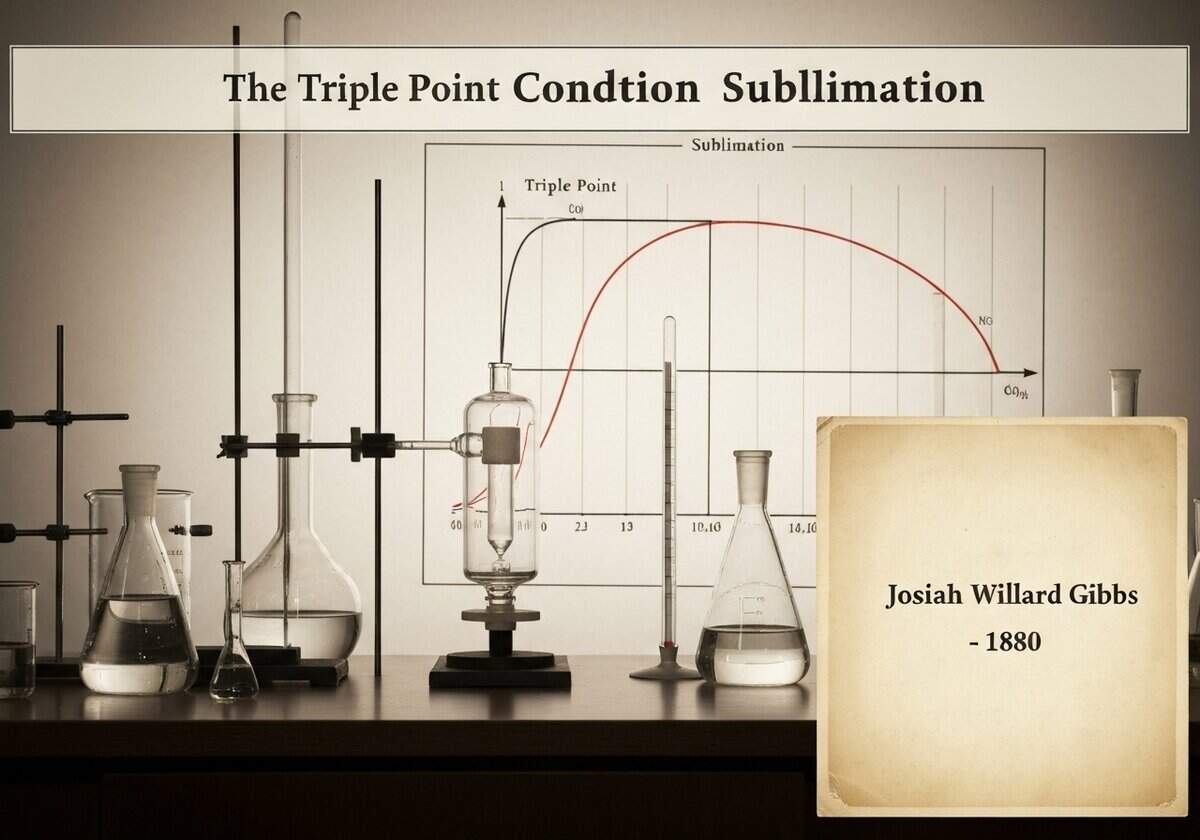
The concept of the triple point is a cornerstone of thermodynamics, formally described by Josiah Willard Gibbs’ phase rule. The phase rule, [latex]F = C – P + 2[/latex], relates the number of degrees of freedom (F) of a system to the number of components (C) and the number of phases (P) in equilibrium. For a pure substance (C=1), if three phases coexist (P=3), the number of degrees of freedom is F = 1 – 3 + 2 = 0. This means the state is invariant; it can only exist at a single, specific combination of temperature and pressure, which is the triple point.
The phase diagram of a substance graphically represents the conditions under which its different phases are stable. It is typically a plot of pressure versus temperature. The diagram is divided into solid, liquid, and gas regions by phase boundaries. The line separating the solid and gas regions is the sublimation curve. Any point on this line represents a state where the solid and gas phases are in equilibrium. If one starts in the solid phase region and lowers the pressure at a constant temperature, crossing this line will induce sublimation. Similarly, heating a solid at a constant pressure that is below the triple point pressure will also cause it to sublime. For water, the triple point is at 0.01 °C (273.16 K) and a pressure of 611.657 Pa. Any pressure below this value will cause ice to sublime directly into water vapor upon heating, bypassing the liquid water phase entirely. This principle is fundamental to processes like freeze-drying, where low pressure is applied to frozen materials to remove water content efficiently without the damaging effects of high heat.
- 化学气相沉积(CVD), 环境影响, 相位图, 工艺优化, 热力学
类型
中断
使用方法
前体
- studies on the states of matter by antoine lavoisier
- development of the kinetic theory of gases by clausius and maxwell
- formulation of the laws of thermodynamics
- early observations of phase changes, such as the boiling of water and melting of ice
应用
- freeze-drying (lyophilization) of food and pharmaceuticals
- understanding comet tail formation in the vacuum of space
- frost formation (deposition)
- purification of chemical compounds via vacuum sublimation
- manufacturing of dry ice
专利:
迎接新挑战
机械工程师、项目、工艺工程师或研发经理
可在短时间内接受新的挑战。
通过 LinkedIn 联系我
塑料金属电子集成、成本设计、GMP、人体工程学、中高容量设备和耗材、精益制造、受监管行业、CE 和 FDA、CAD、Solidworks、精益西格玛黑带、医疗 ISO 13485
相关发明、创新和技术原理














