A systematic process for identifying, documenting, and addressing existing nonconformities (Corrective Action) and potential future nonconformities (Preventive Action) to improve quality and compliance.
- Methodologies: Engineering, Quality
CAPA (Corrective and Preventive Action)

CAPA (Corrective and Preventive Action)
- Continuous Improvement, Corrective Action, Process Improvement, Quality Assurance, Quality Control, Quality Management, Risk Management, Root Cause Analysis, Verification
Objective:
How it’s used:
- Involves investigating the root cause of an identified problem, implementing actions to correct it and prevent recurrence. For preventive action, potential problems are identified and steps are taken to prevent them from occurring in the first place. Includes verification of effectiveness.
Pros
- Leads to sustained quality improvement by addressing root causes; Helps meet regulatory requirements (e.g., ISO, FDA); Prevents recurrence of problems and occurrence of potential problems; Improves customer satisfaction.
Cons
- Can be bureaucratic and time-consuming if not managed efficiently; Requires thorough root cause analysis, which can be complex; Effectiveness depends on proper implementation and follow-up; May require significant resources for investigation and action.
Categories:
- Lean Sigma, Manufacturing, Quality, Risk Management
Best for:
- Addressing and preventing product and process non-conformances, meeting regulatory standards, and driving continuous improvement.
The CAPA methodology is frequently utilized across diverse sectors such as pharmaceuticals, biotechnology, medical devices, automotive, and manufacturing, contributing to enhancing product quality, safety, and compliance with industry standards. Techniques used within CAPA often involve Quality Management Systems (QMS) and Six Sigma methodologies to analyze failures systematically, ensuring that corrective actions are not only thorough but also actionable and measurable. Typically, this methodology is initiated by quality assurance teams or engineers who have identified a defect, non-conformance, or potential risk during product testing or customer feedback analysis, necessitating cross-functional collaboration with R&D, manufacturing, and supply chain stakeholders. Common applications include addressing product recalls, analyzing customer complaints, or building risk mitigation plans for foreseeable issues, reinforcing a culture of quality assurance that aligns with regulatory expectations like those imposed by the FDA or ISO standards. Evaluating the effectiveness of implemented actions is an ongoing process, often involving follow-up audits or metrics assessments, thereby fostering a proactive stance towards quality management that can lead to enhanced customer satisfaction and loyalty while enabling organizations to sustain continuous improvements in their operational processes and product offerings. Engaging employees at all levels in CAPA initiatives promotes accountability and encourages a shared commitment to excellence within the organization, ultimately leading to a more resilient product development life cycle that minimizes waste and optimizes resource allocation.
Key steps of this methodology
- Identify and document the problem or non-conformance.
- Conduct a root cause analysis to determine the underlying reasons.
- Develop corrective action plans to address the identified root causes.
- Implement corrective actions and monitor for effectiveness.
- Evaluate the effectiveness of corrective actions and assess impacts.
- Identify potential issues and develop preventive action plans.
- Implement preventive actions and verify their effectiveness.
- Monitor results and review processes for continuous improvement.
Pro Tips
- Utilize advanced root cause analysis techniques such as Fishbone diagrams or the 5 Whys to identify underlying issues comprehensively.
- Integrate CAPA processes with other quality management systems to ensure cross-functional collaboration and real-time feedback loops.
- Employ data analytics to predict potential non-conformances, allowing for proactive measures and increasing efficiency in preventive actions.
To read and compare several methodologies, we recommend the
> Extensive Methodologies Repository <
together with the 400+ other methodologies.
Your comments on this methodology or additional info are welcome on the comment section below ↓ , so as any engineering-related ideas or links.
Historical Context
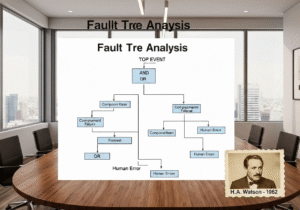
1962
1970
1972
1980
1980
1986
1986
1960
1963
1970
1980
1980
1980
1986
1987
(if date is unknown or not relevant, e.g. "fluid mechanics", a rounded estimation of its notable emergence is provided)











Related Posts
Master Production Schedule (MPS)
Mass Customization
Marketing Funnel
Marketing Audit
MAPO Index (Movement and Assistance of Hospital Patients)
Manufacturing Resource Planning (MRP II)