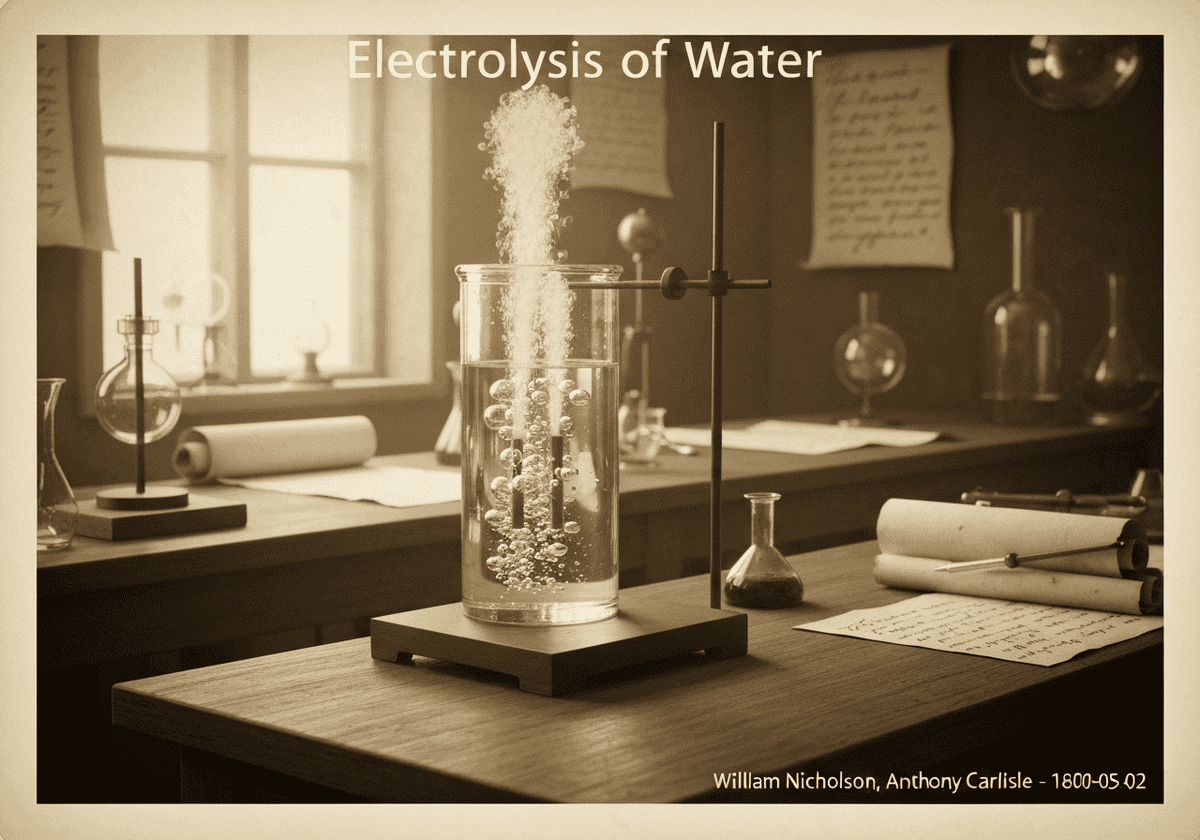
L'électrolyse de l'eau est la décomposition de l'eau (H₂O) en ses éléments constitutifs, l'oxygène (O₂) et l'hydrogène (H₂), par passage d'un courant électrique. À la cathode, deux molécules d'eau sont réduites pour former de l'hydrogène gazeux et des ions hydroxyde. À l'anode, deux molécules d'eau sont oxydées pour former de l'oxygène gazeux, des protons et des électrons.