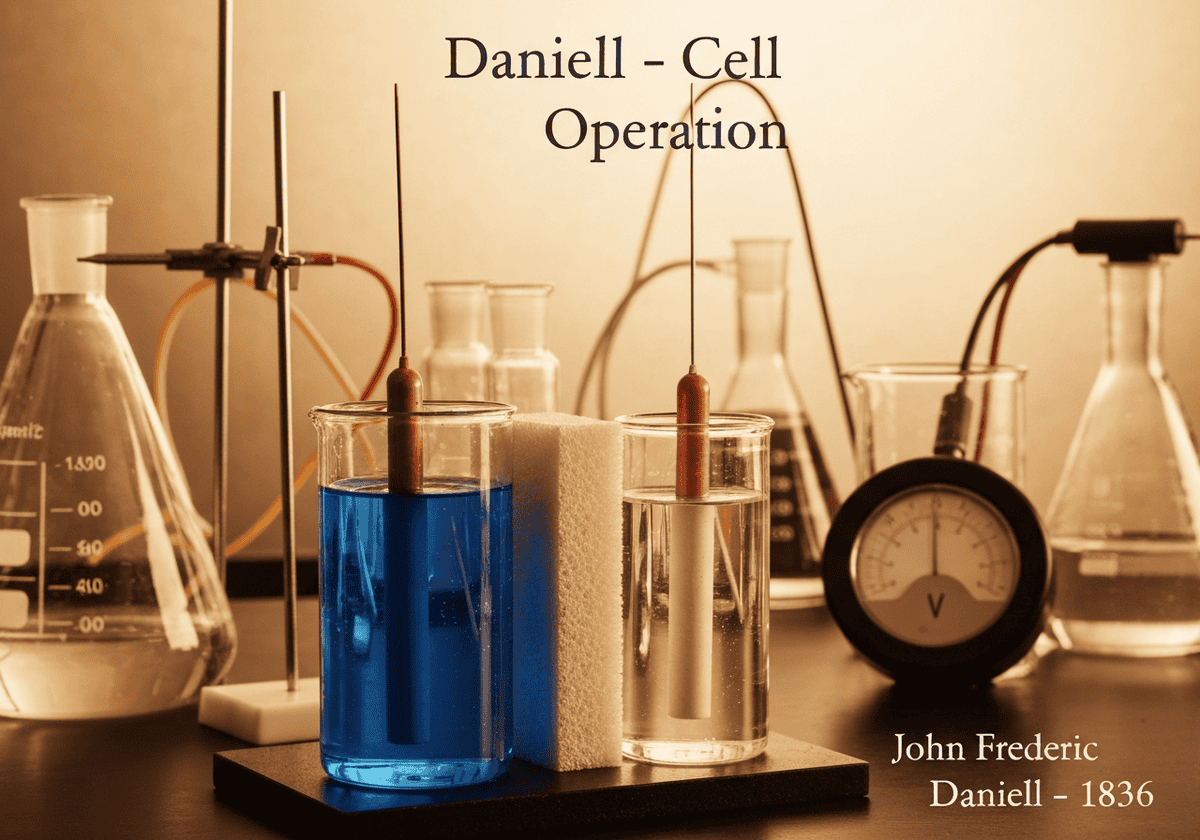
An improvement on the Voltaic pile, the Daniell cell consists of a copper electrode in a copper(II) sulfate solution and a zinc electrode in a zinc sulfate solution, separated by a porous barrier. This two-fluid design prevents hydrogen gas buildup (polarization) on the copper electrode, resulting in a much more stable and reliable voltage source for a longer duration.