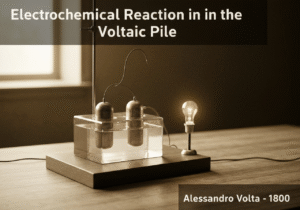
La primera batería eléctrica produce corriente continua mediante la superposición de pares de discos metálicos diferentes (p. ej., zinc y cobre) separados por una tela empapada en salmuera. Cada par forma una celda galvánica, y su superposición en serie aumenta el voltaje total. Esta disposición demostró la primera conversión continua de energía química en energía eléctrica, allanando el camino para la electroquímica moderna.
La pila voltaica funciona según el principio fundamental de la serie electroquímica. Cuando dos metales diferentes, como el zinc y el cobre, se conectan mediante un electrolito (en este caso, papel empapado en salmuera), se produce una reacción electroquímica. El zinc, al ser más reactivo, se oxida fácilmente, perdiendo electrones y formando iones de zinc que se disuelven en el electrolito. Estos electrones viajan por la vía metálica externa hasta el disco de cobre. En la superficie del cobre, se produce una reacción de reducción; normalmente, los iones de hidrógeno del agua del electrolito aceptan los electrones para formar hidrógeno gaseoso. Este flujo de electrones constituye una corriente eléctrica.
Each zinc-copper pair, separated by the electrolyte, is a single ‘cell’ that produces a small voltage (around 0.76 volts). The genius of Volta’s design was stacking these cells in series. By placing them one on top of another (copper, zinc, brine-soaked cloth, copper, zinc, etc.), the voltages of the individual cells add up. A pile with 20 cells could produce about 15 volts. This was the first device to provide a steady, continuous source of electric current, unlike the static discharges from Leyden jars.
However, the Voltaic pile had significant limitations. The hydrogen gas produced at the copper electrode would form a layer of bubbles, insulating the electrode from the electrolyte. This phenomenon, known as polarization, quickly increased the internal resistance and caused the voltage to drop. Additionally, the electrolyte would leak and evaporate, and local short-circuits could occur, limiting its practical esperanza de vida.