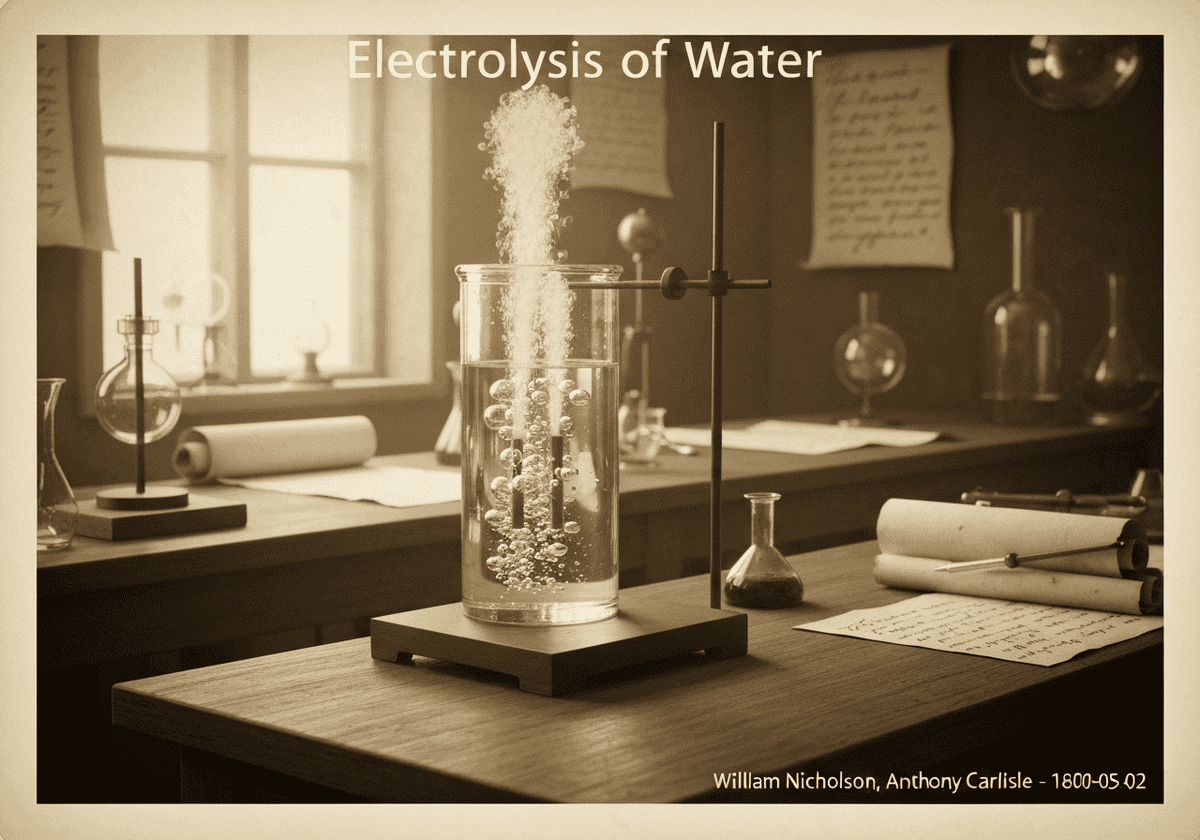
La electrólisis del agua consiste en su descomposición en sus elementos constituyentes, oxígeno (O₂) e hidrógeno (H₂), mediante el paso de una corriente eléctrica. En el cátodo, dos moléculas de agua se reducen para formar hidrógeno gaseoso e iones hidróxido. En el ánodo, dos moléculas de agua se oxidan para formar oxígeno gaseoso, protones y electrones.