Ein systematischer Prozess zur Identifizierung, Dokumentation und Behebung bestehender Nichtkonformitäten (Korrekturmaßnahmen) und potenzieller zukünftiger Nichtkonformitäten (Vorbeugungsmaßnahmen) zur Verbesserung der Qualität und Einhaltung der Vorschriften.
- Methodologien: Projektmanagement
CAPA (Korrektur- und Vorbeugungsmaßnahmen)

CAPA (Korrektur- und Vorbeugungsmaßnahmen)
- Kontinuierliche Verbesserung, Korrekturmaßnahme, Prozessverbesserung, Qualitätssicherung, Qualitätskontrolle, Qualitätsmanagement, Risikomanagement, Ursachenanalyse, Überprüfung
Zielsetzung:
Wie es verwendet wird:
- Es geht darum, die Ursache eines festgestellten Problems zu untersuchen, Maßnahmen zu seiner Behebung zu ergreifen und ein erneutes Auftreten zu verhindern. Bei Präventivmaßnahmen werden potenzielle Probleme ermittelt und Schritte unternommen, um zu verhindern, dass sie überhaupt auftreten. Umfasst auch die Überprüfung der Wirksamkeit.
Vorteile
- Führt zu einer nachhaltigen Qualitätsverbesserung durch Beseitigung der Ursachen; hilft bei der Erfüllung gesetzlicher Vorschriften (z. B. ISO, FDA); verhindert das Wiederauftreten von Problemen und das Auftreten potenzieller Probleme; verbessert die Kundenzufriedenheit.
Nachteile
- Kann bürokratisch und zeitaufwändig sein, wenn es nicht effizient gehandhabt wird; Erfordert eine gründliche Ursachenanalyse, die komplex sein kann; Die Wirksamkeit hängt von der ordnungsgemäßen Umsetzung und Weiterverfolgung ab; Kann erhebliche Ressourcen für Untersuchungen und Maßnahmen erfordern.
Kategorien:
- Lean Sigma, Herstellung, Qualität, Risikomanagement
Am besten geeignet für:
- Umgang mit und Vermeidung von Produkt- und Prozessabweichungen, Einhaltung gesetzlicher Normen und kontinuierliche Verbesserung.
The CAPA methodology is frequently utilized across diverse sectors such as pharmaceuticals, biotechnology, medical devices, automotive, and manufacturing, contributing to enhancing product quality, safety, and compliance with industry standards. Techniques used within CAPA often involve Quality Management Systems (QMS) and Six Sigma methodologies to analyze failures systematically, ensuring that corrective actions are not only thorough but also actionable and measurable. Typically, this methodology is initiated by quality assurance teams or engineers who have identified a defect, non-conformance, or potential risk during product testing or customer feedback analysis, necessitating cross-functional collaboration with R&D, manufacturing, and supply chain stakeholders. Common applications include addressing product recalls, analyzing customer complaints, or building risk mitigation plans for foreseeable issues, reinforcing a culture of quality assurance that aligns with regulatory expectations like those imposed by the FDA or ISO standards. Evaluating the effectiveness of implemented actions is an ongoing process, often involving follow-up audits or metrics assessments, thereby fostering a proactive stance towards quality management that can lead to enhanced customer satisfaction and loyalty while enabling organizations to sustain continuous improvements in their operational processes and product offerings. Engaging employees at all levels in CAPA initiatives promotes accountability and encourages a shared commitment to excellence within the organization, ultimately leading to a more resilient product development Lebenszyklus that minimizes waste and optimizes resource allocation.
Die wichtigsten Schritte dieser Methodik
- Identify and document the problem or non-conformance.
- Conduct a root cause analysis to determine the underlying reasons.
- Develop corrective action plans to address the identified root causes.
- Implement corrective actions and monitor for effectiveness.
- Evaluate the effectiveness of corrective actions and assess impacts.
- Identify potential issues and develop preventive action plans.
- Implement preventive actions and verify their effectiveness.
- Monitor results and review processes for continuous improvement.
Profi-Tipps
- Utilize advanced root cause analysis techniques such as Fishbone diagrams or the 5 Warums to identify underlying issues comprehensively.
- Integrate CAPA processes with other quality management systems to ensure cross-functional collaboration and real-time feedback loops.
- Employ data analytics to predict potential non-conformances, allowing for proactive measures and increasing efficiency in preventive actions.
Verschiedene Methoden lesen und vergleichen, Wir empfehlen die
> Umfassendes Methoden-Repository <
zusammen mit den über 400 anderen Methoden.
Ihre Kommentare zu dieser Methodik oder zusätzliche Informationen sind willkommen auf der Kommentarbereich unten ↓ , sowie alle ingenieursbezogenen Ideen oder Links.
Historischer Kontext
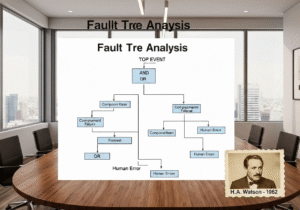
1962
1970
1972
1980
1980
1986
1986
1960
1963
1970
1980
1980
1980
1986
1987
(wenn das Datum nicht bekannt oder nicht relevant ist, z. B. "Strömungsmechanik", wird eine gerundete Schätzung des bemerkenswerten Erscheinens angegeben)











Verwandte Artikel
Fragebögen zu muskuloskelettalen Beschwerden
Multivariate Tests (MVT)
Mehrfache Regressionsanalyse
Motion-Capture-Systeme
MoSCoW-Methode
Moods Median-Test