To systematically collect and organize data in real-time, often at the location where the data is generated, making it easy to observe patterns and frequencies of occurrences.
- Méthodologies : Clients et marketing, Économie, Conception de Produits
Feuilles de contrôle

Feuilles de contrôle
- Amélioration continue, Production allégée, Techniques de résolution de problèmes, Amélioration des processus, Contrôle de qualité, Gestion de la qualité, Analyse des causes profondes, Contrôle statistique des processus (CSP)
Objectif :
Comment il est utilisé :
- A simple, pre-designed form used to tally or mark the occurrences of specific events, defects, or conditions. Marks are made against predefined categories as observations are made.
Avantages
- Easy to use and understand; Provides a clear, visual record of data; Helps in identifying the frequency of problems or events directly at the source; Low cost to implement.
Inconvénients
- Limited to collecting data on predefined categories (may miss new issues); Does not inherently provide reasons for occurrences; Can be prone to human error in recording if not carefully managed.
Catégories :
- Lean Sigma, Fabrication, Résolution de problèmes, Qualité
Idéal pour :
- Tracking defects, monitoring process steps, collecting frequency data for specific issues, and initial data gathering for problem identification.
Check sheets can be applied effectively in various stages of product design and development processes, particularly during quality assurance and process improvement phases. Industries such as manufacturing, healthcare, and software development utilize check sheets for defect tracking and process monitoring. In manufacturing, a check sheet may document occurrences of defects on the production line, allowing teams to pinpoint areas in need of immediate corrective action. In healthcare, check sheets can assist in tracking patient care issues or medication errors, ensuring a systematic approach to identifying patterns that may require intervention. When developing software, check sheets can be beneficial for recording bug occurrences or feature requests during testing phases. This methodology is usually initiated by quality control teams or project managers but can also involve input from all stakeholders engaged in the design process, including engineers and end users. The ease of creating and interpreting check sheets empowers teams to collect and analyze data quickly, facilitating informed decision-making by highlighting prevalent issues within the workflow. Their inherent simplicity encourages broader participation across departments, making this approach a collaborative effort in identifying and addressing challenges in product design and innovation initiatives.
Principales étapes de cette méthodologie
- Define the categories of events or defects to be tracked.
- Create the Check Sheet format with predefined columns for each category.
- Establish criteria for marking occurrences accurately.
- Implement the Check Sheet in the relevant process or area.
- Regularly review and update the Check Sheet based on observations.
- Analyze patterns and trends from the tallied data for further action.
- Refine tracking methods based on findings from the data analysis.
Conseils de pro
- Incorporate a digital tool or software for real-time updates and analysis to enhance data accuracy and reduce human error.
- Utilize statistical analysis to interpret data trends gathered from Check Sheets, informing targeted interventions for recurring issues.
- Regularly review and revise Check Sheet categories based on evolving processes or product changes to maintain relevance and effectiveness in data collection.
Lire et comparer plusieurs méthodologies, nous recommandons le
> Référentiel méthodologique étendu <
ainsi que plus de 400 autres méthodologies.
Vos commentaires sur cette méthodologie ou des informations supplémentaires sont les bienvenus sur le site web de la Commission européenne. section des commentaires ci-dessous ↓ , ainsi que toute idée ou lien en rapport avec l'ingénierie.
Contexte historique
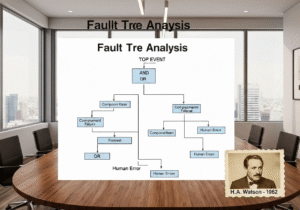
1962
1970
1972
1980
1980
1986
1986
1960
1963
1970
1980
1980
1980
1986
1987
(si la date est inconnue ou n'est pas pertinente, par exemple "mécanique des fluides", une estimation arrondie de son émergence notable est fournie)











Articles Similaires
Programme directeur de production (PDP)
Personnalisation de masse
Entonnoir marketing
Audit marketing
Indice MAPO (Mouvement et assistance des patients hospitalisés)
Planification des ressources de fabrication (MRP II)