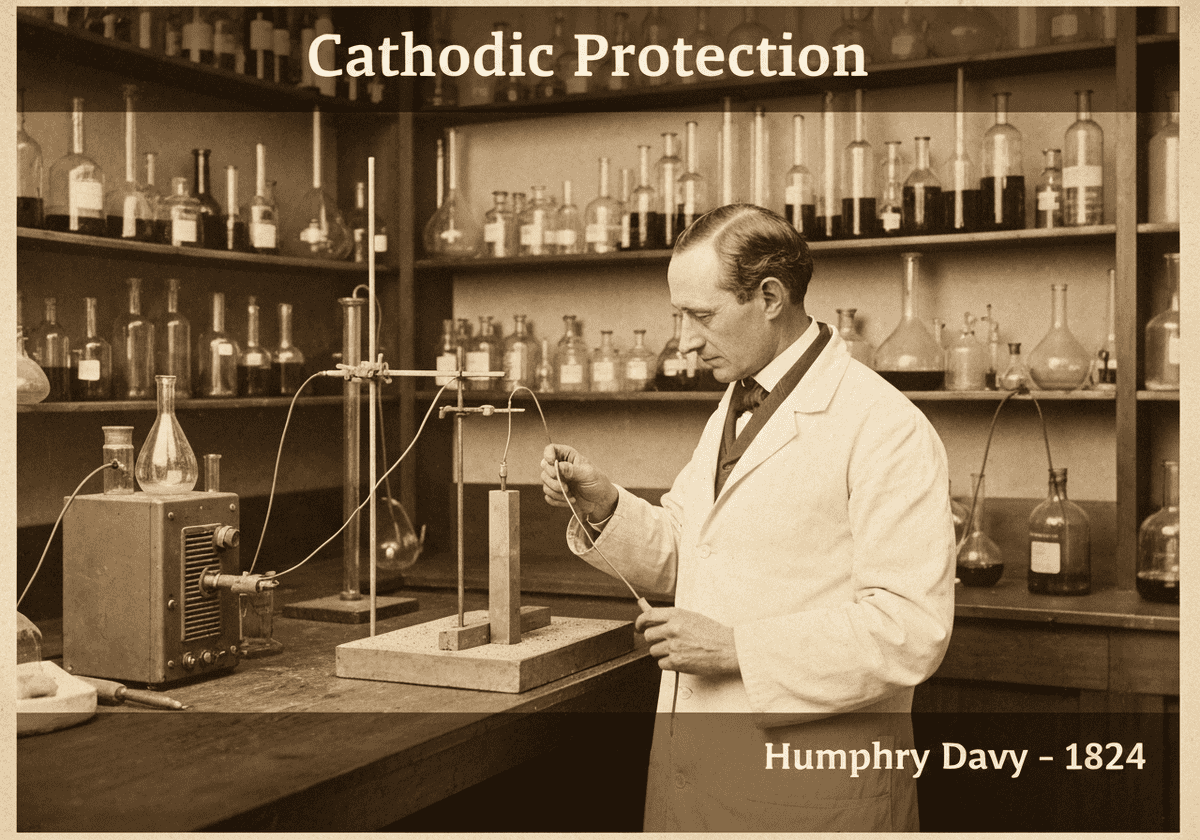
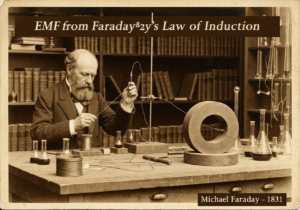
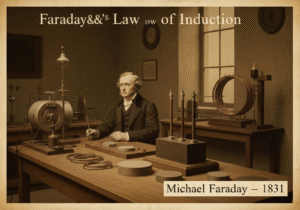
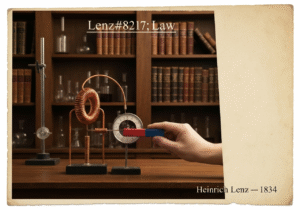
Cathodic protection (CP) is a technique used to control the corrosion of a metal surface by making it the cathode of an electrochemical cell. This is achieved by supplying an external electrical current that suppresses the natural corrosion currents. The protected metal’s potential is polarized to a more negative value, shifting it into a region of immunity or passivity.