A document that describes the systems and processes used to control product and process quality to ensure customer requirements are met.
- Methodologien: Maschinenbau, Qualität
Kontrollplan

Kontrollplan
- Erweiterte Produktqualitätsplanung (APQP), Kontrollkarte, Korrekturmaßnahme, Prozessverbesserung, Qualitätssicherung, Qualitätskontrolle, Qualitätsmanagement, Qualitätsmanagementsystem (QMS), Statistische Prozesskontrolle (SPC)
Zielsetzung:
Wie es verwendet wird:
- Used in manufacturing (often as part of APQP), it is a living document that outlines the measurements, inspections, and checks that will be performed at each stage of the process to ensure output is consistent.
Vorteile
- Provides a structured and standardized approach to quality control, ensures all critical characteristics are monitored, and serves as a key reference document for production staff.
Nachteile
- Can be bureaucratic and time-consuming to create and maintain, requires discipline to follow consistently, and may not be flexible enough to handle unexpected process variations.
Kategorien:
- Maschinenbau, Lean Sigma, Herstellung, Qualität
Am besten geeignet für:
- Documenting the specific quality control methods and actions used to control a manufacturing process and ensure product quality.
The Control Plan methodology is widely utilized across industries such as automotive, aerospace, electronics, and medical devices, where ensuring product quality is paramount. In the context of Advanced Product Quality Planning (APQP), it is particularly relevant during the design and development phase, as well as during ongoing production, encompassing all stages from initial design validation to final assembly. Key participants in developing and maintaining a Control Plan typically include quality engineers, manufacturing engineers, process managers, and cross-functional team members, who collaborate to ensure that all significant process variables are defined and monitored systematically. The Control Plan is inherently a living document, meaning it must be continuously updated based on process changes, improved technologies, or new customer requirements, thereby facilitating a proactive approach to quality management rather than a reactive one. Typical applications include outlining specific measurement techniques such as Statistische Prozesskontrolle (SPC), capability studies, and inspection methods that ensure compliance with design specifications. The use of this methodology not only enhances traceability and accountability in manufacturing processes but also serves as a training resource for new employees, reinforcing the organization’s commitment to maintaining high quality standards throughout the production lifecycle. Additionally, the alignment of the Control Plan with other quality management tools, such as Failure Mode and Effects Analysis (FMEA) and Design Reviews, promotes a holistic approach to quality assurance, thus increasing customer satisfaction and reducing product costs associated with defects and rework.
Die wichtigsten Schritte dieser Methodik
- Identify critical characteristics for the product and process.
- Develop measurement criteria and methods for each characteristic.
- Establish control methods and inspection frequencies.
- Document responsibilities for monitoring and actions taken.
- Integrate feedback loops for continuous improvement.
- Review and update the Control Plan regularly based on process changes.
Profi-Tipps
- Regularly update the Control Plan as changes occur in materials, processes, or equipment, ensuring alignment with the latest production standards.
- Integrate real-time data collection and analysis tools within the Control Plan to enhance decision-making and prompt corrective actions during the manufacturing process.
- Include a robust training section within the Control Plan for personnel, emphasizing the importance of adherence to quality checks and continuous improvement procedures.
Verschiedene Methoden lesen und vergleichen, Wir empfehlen die
> Umfassendes Methoden-Repository <
zusammen mit den über 400 anderen Methoden.
Ihre Kommentare zu dieser Methodik oder zusätzliche Informationen sind willkommen auf der Kommentarbereich unten ↓ , sowie alle ingenieursbezogenen Ideen oder Links.
Historischer Kontext
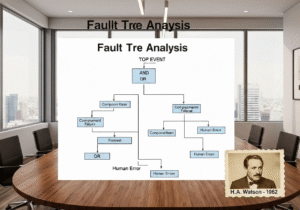
1962
1970
1972
1980
1980
1986
1986
1960
1963
1970
1980
1980
1980
1986
1987
(wenn das Datum nicht bekannt oder nicht relevant ist, z. B. "Strömungsmechanik", wird eine gerundete Schätzung des bemerkenswerten Erscheinens angegeben)











Verwandte Artikel
METS-Kalorien-Rechner
Meta-Analyse
Nachrichten-Mapping
Mentalmodell-Diagramme
Maximal zulässige Druck- und Zugkräfte
Materialbedarfsplanung (MRP)