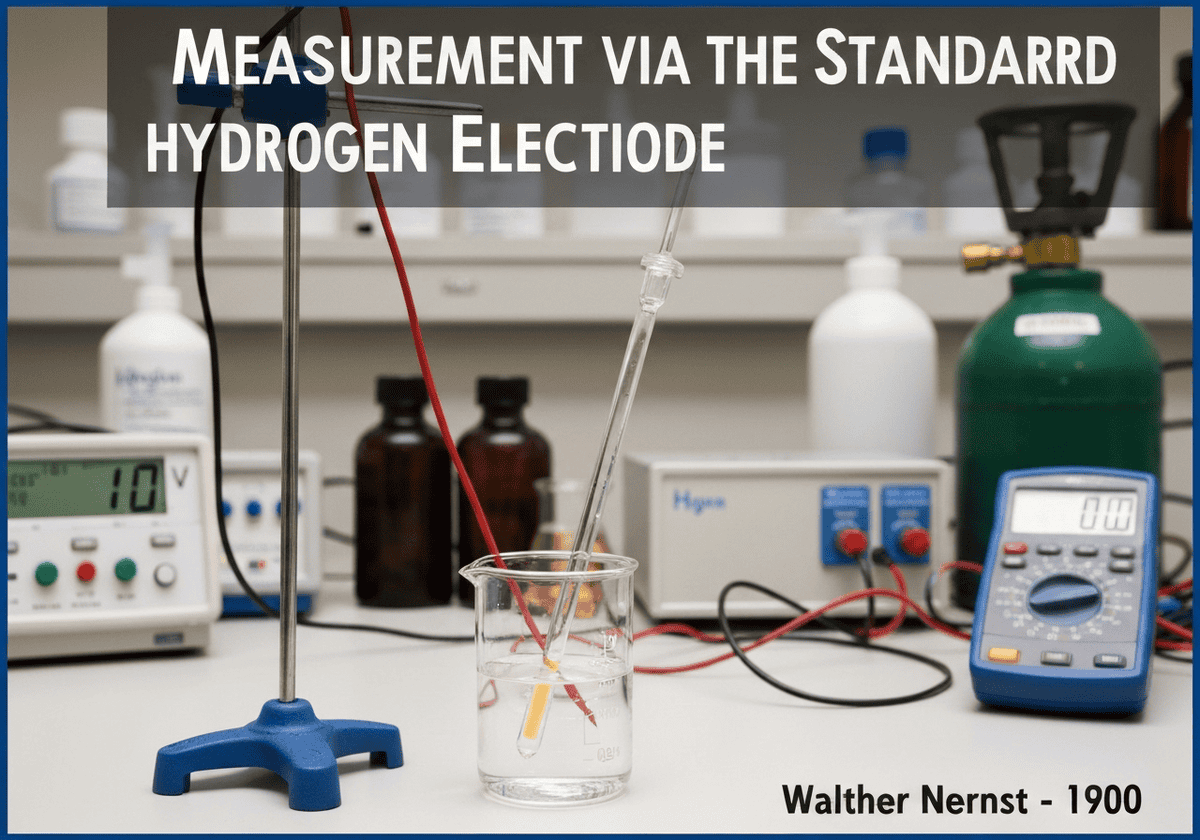
The absolute electrochemical potential of a single electrode cannot be measured. Instead, potentials are measured relative to a universally accepted reference. The Standard Hydrogen Electrode (SHE) is the primary reference, assigned a potential of exactly 0 volts under standard conditions (1 M [latex]H^+[/latex] concentration, 1 bar [latex]H_2[/latex] gas, 298 K). All other standard electrode potentials are reported relative to this zero point.