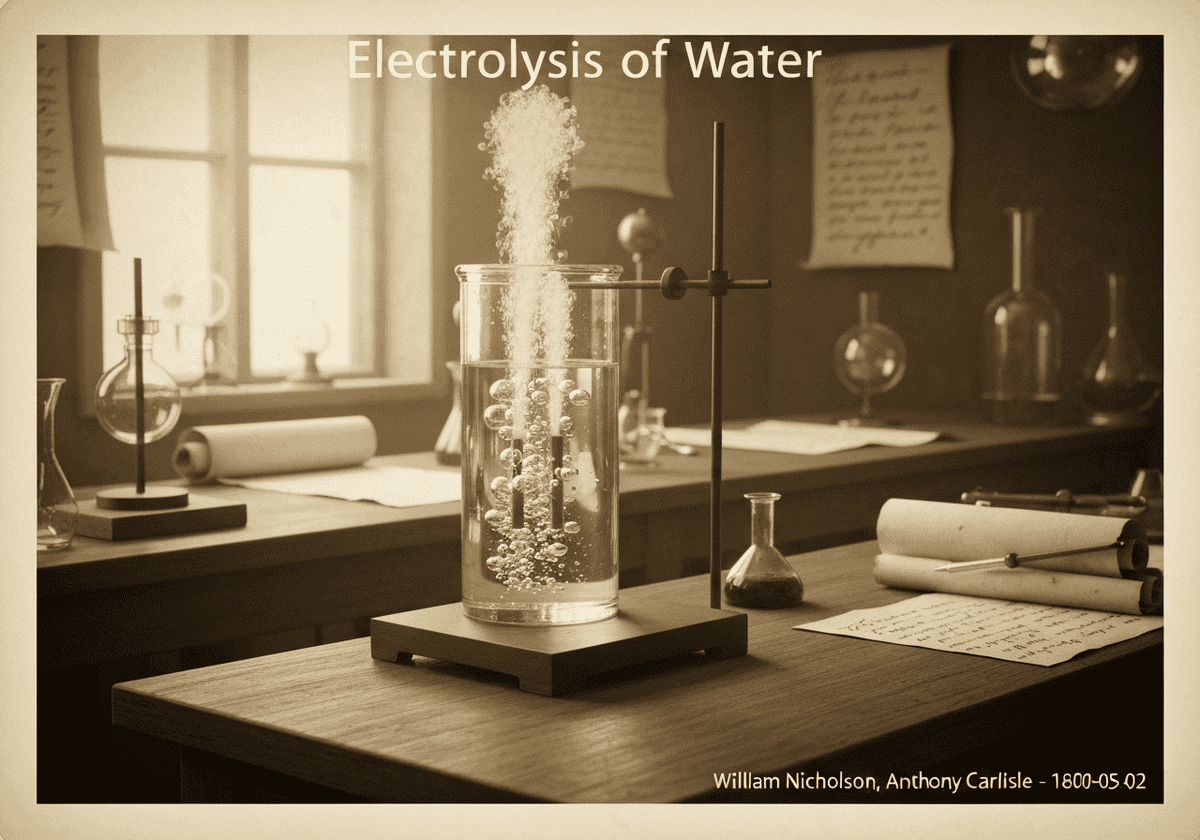
水的电解是通过电流通过水(H₂O)分解成其组成元素氧气(O₂)和氢气(H₂)。在阴极,两个水分子被还原形成氢气和氢氧根离子。在阳极,两个水分子被氧化形成氧气、质子和电子。

(generate image for illustration only)
水的电解是通过电流通过水(H₂O)分解成其组成元素氧气(O₂)和氢气(H₂)。在阴极,两个水分子被还原形成氢气和氢氧根离子。在阳极,两个水分子被氧化形成氧气、质子和电子。
The decomposition of pure water into hydrogen and oxygen is a thermodynamically unfavorable process, requiring external energy in the form of electricity. Pure water is a poor conductor of electricity, so an electrolyte, such as a small amount of a soluble salt or an acid like sulfuric acid, is typically added to increase conductivity. The overall balanced equation for the reaction is [latex]2H_2O(l) \rightarrow 2H_2(g) + O_2(g)[/latex].
The process occurs in an electrolytic cell. At the negatively charged cathode, a reduction reaction takes place: [latex]2H_2O(l) + 2e^- \rightarrow H_2(g) + 2OH^-(aq)[/latex]. At the positively charged anode, an oxidation reaction occurs: [latex]2H_2O(l) \rightarrow O_2(g) + 4H^+(aq) + 4e^-[/latex]. The production of hydrogen gas at the cathode is exactly twice the volume of oxygen gas produced at the anode, a direct consequence of the stoichiometry of water. This 2:1 volume ratio is a classic demonstration in chemistry classrooms, often performed using a Hofmann voltameter.
The minimum voltage required for electrolysis to occur, known as the decomposition potential, is 1.23 V at standard conditions. However, in practice, a higher voltage, called 过电位, is needed to overcome various activation barriers. The efficiency of water electrolysis is a key factor in the viability of a hydrogen-based economy, with significant research focused on developing more effective and cheaper catalysts for the anode and cathode to reduce the overpotential and energy consumption.
迎接新挑战
机械工程师、项目、工艺工程师或研发经理
可在短时间内接受新的挑战。
通过 LinkedIn 联系我
塑料金属电子集成、成本设计、GMP、人体工程学、中高容量设备和耗材、精益制造、受监管行业、CE 和 FDA、CAD、Solidworks、精益西格玛黑带、医疗 ISO 13485
水的电解
(如果日期不详或不相关,例如 "流体力学",则对其显著出现的时间作了四舍五入的估计)。
相关发明、创新和技术原理
{{标题}}
{%,如果摘录 %}{{ 摘录 | truncatewords:55 }}
{% endif %}