In the domains of product design, engineering, manufacturing, and ابتكار, the evaluation of the risk-benefit ratio in risk assessment plays a big role in decision-making processes that safeguard human life and promote sustainable practices. A statistic from the World Health Organization underscores this urgency: approximately 1 in 10 patients worldwide experience adverse effects from medication, highlighting the need for rigorous risk assessment in pharmaceutical drug development and جهاز طبي design. This article aims to dissect the multifaceted definition of the risk-benefit ratio, the methodologies for its evaluation -both qualitative and quantitative- as well as the key factors influencing this ratio.
Ethical considerations in Risk–benefit Ratio In Risk Assessment and the relevant regulatory and legal frameworks will also be discussed, supported by real-world case studies that illustrate the practical applications and challenges faced when assessing risk against potential benefits.
النقاط الرئيسية
- Risk-benefit ratio quantifies potential outcomes in assessments.
- Evaluation methods include both qualitative and quantitative analysis.
- Severity and likelihood of risks significantly influence the ratio.
- Ethical frameworks guide decision-making processes around risks.
- Understanding regulatory requirements is crucial for compliance.
- Case studies illustrate practical applications and challenges faced.

Definition of Risk-Benefit Ratio in Risk Assessment
The concept of risk-benefit ratio serves as a fundamental نطاق in risk assessment across various industries, especially when weighing the potential adverse effects against the anticipated benefits of a decision or action. It quantitatively represents how much benefit is gained versus the possible risks associated with a particular course of action. For instance, in pharmaceutical drug development, the ratio helps determine whether a new medication’s therapeutic advantages outweigh its side effects and potential for harm. A favorable risk-benefit ratio supports the approval and use of a drug, guiding regulatory bodies like the FDA in their assessments and market authorization.
This ratio is typically expressed in quantitative terms, rendering it easier to compare different options. Mathematically, it can be represented basically as [latex]RBR = \frac{Benefit}{Risk}[/latex].
If the calculated RBR indicates that benefits substantially exceed risks, stakeholders may consider proceeding with the project. Conversely, a lower or negative ratio may lead to reconsideration or abandonment. This formula aids in making data-driven decisions rather than relying solely on subjective judgments.
Key elements contributing to the risk-benefit ratio include the nature and severity of risks involved, the likelihood of their occurrence, and the extent of the benefits derived:
- In engineering project management, a construction project that promises significant economic advantages but poses severe safety risks may reveal a less favorable risk-benefit ratio.
- A project with numerous minor risks but moderate benefits might show a more attractive ratio.
Real-world metrics can further elucidate this concept. In medical device design, a device designed to reduce surgical time but poses risks of infection with a 5% probability yields a different RBR compared to another device with lower infection risk but minimal time-saving effects. Thus, the risk-benefit ratio not only guides regulatory approvals but shapes strategic planning within projects across different sectors.
نصيحة: Utilize decision trees to visualize the consequences of various actions in relation to their risk-benefit ratios, aiding in clearer decision-making.
Methodology for Evaluating Risk-Benefit Ratio
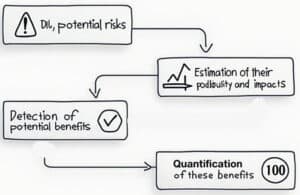
Evaluating the risk-benefit ratio involves several systematic approaches that aim to quantify both the risks and the benefits of a decision or action. A common methodology includes the following steps:
- identification of potential risks
- estimation of their probabilities and impacts
- detection of potential benefits
- quantification of these benefits
In pharmaceutical drug development, methodologies such as failure mode and effects analysis (FMEA) are often employed. This allows for the prioritization of risks based on severity and likelihood, guiding decision-makers in attempting to minimize risks while maximizing patient benefits.
Specific metrics and tools can greatly assist in evaluating the risk-benefit ratio. For example, the use of statistical models, like Bayesian analysis, allows for dynamic updating of risk assessments in real-time as more data becomes available. In clinical trials, determining the “Number Needed to Treat” (NNT) provides valuable insight into the efficacy of a drug versus its associated risks, allowing stakeholders to make more informed decisions about drug approval. A reliable NNT value generally falls below 10 for effective therapies.
| Domain | Common Metrics | Risk Assessment طريقة |
|---|---|---|
| Pharmaceutical | NNT, ADR rates | FMEA, Bayesian |
| الأجهزة الطبية | Reliability Index | Quantitative Risk Assessment |
| الهندسة | Cost Overrun Percentage | Risk Matrix |
| Environmental Policy | Pollution Index | دورة الحياة التحليل |
| Financial Investment | العائد على الاستثمار, VaR | محاكاة مونت كارلو |
Key Factors & Ethical Considerations in Risk-Benefit Decision Making
Adverse Effects

The initial factor that comes to mind: the assessment must ensure that the potential benefits of any action or product do not supersede the ethical implications of the risks involved. Ignoring these implications could result in harmful consequences for patients and could lead to significant public backlash against organizations.
Risks can be quantified and categorized in various ways, including frequency of occurrence, severity of outcomes, and the population affected. For instance, in pharmaceutical development, the risk of adverse reactions may vary widely between different populations, leading to a dynamic risk assessment. Evaluating historical data on drug side effects allows for a calculation of expected adverse الأحداث using the formula: [latex]Risk = \frac{Number of adverse events}{Total population exposed}[/latex]. While there is no official threshold, this quantitative analysis facilitates informed decisions regarding drug safety and efficacy.
For example, the introduction of a minimally invasive surgery device may lower recovery times and hospital stays, thus providing substantial economic and personal benefits. Stakeholders must quantify both direct benefits, such as reduced morbidity, and indirect benefits, such as improved productivity leading to more patients treated, to create a comprehensive picture of value.
A MUST DO: company...
You have read 41% of the article. The rest is for our community. Already a member? تسجيل الدخول
(وأيضًا لحماية المحتوى الأصلي لدينا من روبوتات الكشط)
مجتمع الابتكار العالمي
تسجيل الدخول أو التسجيل (100% مجاناً)
اطلع على بقية هذه المقالة وجميع المحتويات والأدوات الخاصة بالأعضاء فقط.
فقط المهندسون والمصنعون والمصممون والمسوقون الحقيقيون المحترفون.
لا روبوت، ولا كاره، ولا مرسل رسائل غير مرغوب فيها.
الأسئلة الشائعة
What is the Definition of Risk-Benefit Ratio in Risk Assessment?
What Methodology is Used for Evaluating Risk-Benefit Ratios?
What are the Key Factors Influencing the Risk-Benefit Ratio?
What Ethical Considerations Affect Risk-Benefit Decision Making?
What are the Regulatory and Legal Aspects of Risk-Benefit Analysis?
How is Risk-Benefit Analysis Applied in Different Sectors?
مواضيع ذات صلة
- Quantitative Risk Assessment: a systematic approach to measure risks using numerical values and statistical methods.
- Qualitative Risk Assessment: a subjective evaluation of risks based on non-numerical data and expert judgement.
- Monte Carlo Simulation: a computational method using random sampling to estimate risk and uncertainty in quantitative analysis.
- Decision Trees: graphical representations that outline potential decisions and their possible consequences in risk evaluation.
- Failure Mode and Effects Analysis (FMEA): a step-by-step approach to identifying potential failure modes and their impact on system performance.
- Cost-Benefit Analysis: a financial assessment comparing the costs of an action to its benefits to evaluate feasibility and efficiency.
- Uncertainty Analysis: the study of the uncertainty in model inputs and how it affects the overall risk assessment outcome.
- Regulatory Impact Analysis: assessing the potential effects of regulations on costs, benefits, and risks associated with compliance.
- Public Health Perspectives: understanding the implications of risk-benefit analysis in healthcare decisions impacting population health.
- Comparative Effectiveness Research: evaluating different interventions to determine which offers the best outcomes relative to risks.
- Post-Market Surveillance: ongoing monitoring of products after they are released to assess real-world performance and risk mitigation.
- Cost-Effectiveness Analysis: comparing the relative expenditures of different interventions to determine their value based on health outcomes.
- Behavioral Risk Assessment: exploring how human behavior influences risk perception and decision-making in product development.
External Links on Risk–benefit Ratio In Risk Assessment
المعايير الدولية
(حرك الرابط لرؤية وصفنا للمحتوى)
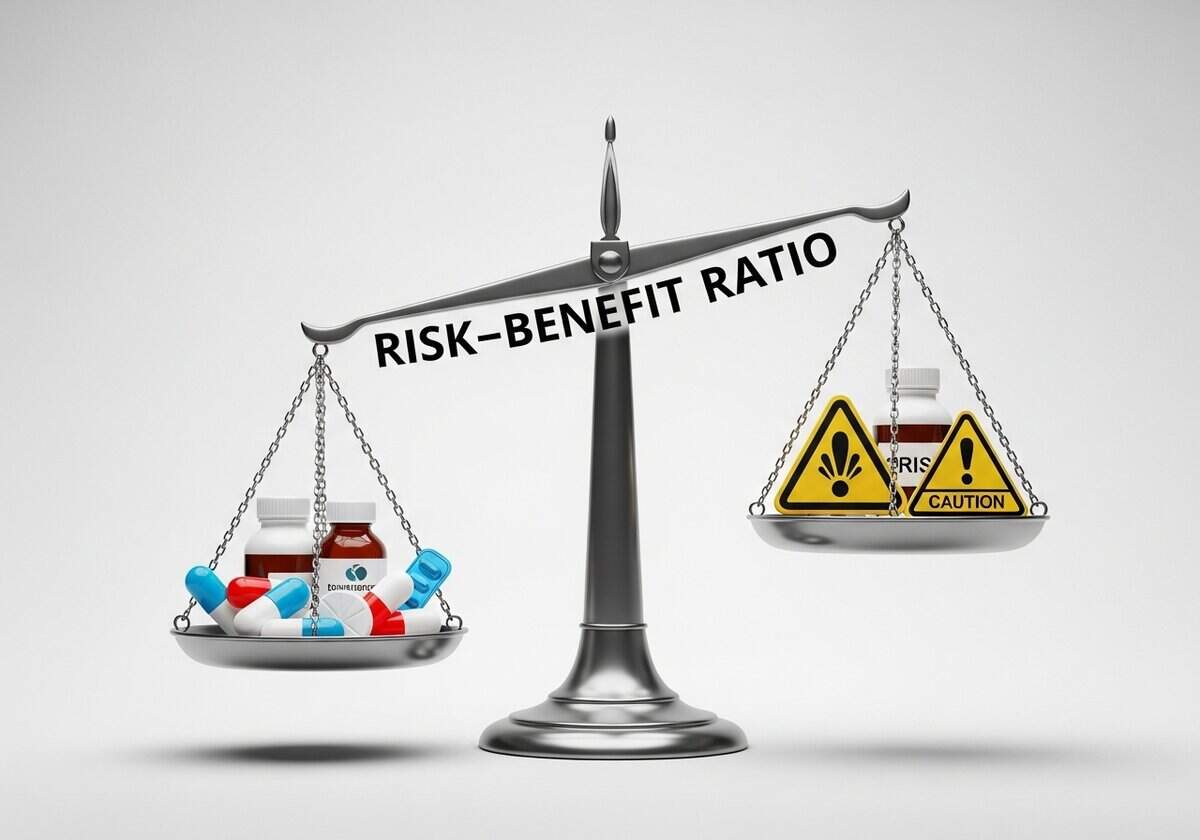











can ethical considerations in risk-benefit decision making can vary greatly depending on cultural perspectives?
needs more on qualitative factors also influencing the assessment
should cover more about the societal impacts on risk-benefit ratio decisions
منشورات ذات صلة
جميع حالات براءات الاختراع: معاهدة التعاون بشأن البراءات مقابل براءة اختراع قيد الانتظار مقابل براءة اختراع منشورة مقابل براءة اختراع ممنوحة
أفضل 10 استراتيجيات وأدوات لإبطال براءات الاختراع
تقييم دورة الحياة (LCA) في تصميم المنتج على وجه التحديد
نظرة عامة على تحليل قيمة المنتج
تقييم بيئة العمل المريحة
أمر التغيير الهندسي (ECO): أفضل الممارسات لتقليل الاضطراب والتكلفة