识别、记录和解决现有不符合项(纠正措施)和未来潜在不符合项(预防措施)的系统流程,以提高质量和合规性。
- 方法: 工程, 质量
CAPA(纠正和预防措施)

CAPA(纠正和预防措施)
- 持续改进, 纠正措施, 流程改进, 质量保证, 质量控制, 质量管理, 风险管理, 根本原因分析, 验证
目标
如何使用
- 包括调查已发现问题的根本原因,采取行动加以纠正并防止再次发生。对于预防性行动,要找出潜在问题,并采取措施防止问题再次发生。包括有效性验证。
优点
- 通过解决根本原因,实现持续的质量改进;帮助满足监管要求(如 ISO、FDA);防止再次出现问题和潜在问题;提高客户满意度。
缺点
- 如果管理效率不高,可能会官僚化,耗费时间;需要进行彻底的根本原因分析,这可能很复杂;有效性取决于适当的实施和跟进;可能需要大量资源用于调查和行动。
类别
- 精益西格玛, 制造业, 质量, 风险管理
最适合:
- 解决并防止产品和流程不合格问题,满足监管标准,推动持续改进。
The CAPA methodology is frequently utilized across diverse sectors such as pharmaceuticals, biotechnology, medical devices, automotive, and manufacturing, contributing to enhancing product quality, safety, and compliance with industry standards. Techniques used within CAPA often involve Quality Management Systems (QMS) and Six Sigma methodologies to analyze failures systematically, ensuring that corrective actions are not only thorough but also actionable and measurable. Typically, this methodology is initiated by quality assurance teams or engineers who have identified a defect, non-conformance, or potential risk during product testing or customer feedback analysis, necessitating cross-functional collaboration with R&D, manufacturing, and supply chain stakeholders. Common applications include addressing product recalls, analyzing customer complaints, or building risk mitigation plans for foreseeable issues, reinforcing a culture of quality assurance that aligns with regulatory expectations like those imposed by the FDA or ISO standards. Evaluating the effectiveness of implemented actions is an ongoing process, often involving follow-up audits or metrics assessments, thereby fostering a proactive stance towards quality management that can lead to enhanced customer satisfaction and loyalty while enabling organizations to sustain continuous improvements in their operational processes and product offerings. Engaging employees at all levels in CAPA initiatives promotes accountability and encourages a shared commitment to excellence within the organization, ultimately leading to a more resilient product development 生命周期 that minimizes waste and optimizes resource allocation.
该方法的关键步骤
- Identify and document the problem or non-conformance.
- Conduct a root cause analysis to determine the underlying reasons.
- Develop corrective action plans to address the identified root causes.
- Implement corrective actions and monitor for effectiveness.
- Evaluate the effectiveness of corrective actions and assess impacts.
- Identify potential issues and develop preventive action plans.
- Implement preventive actions and verify their effectiveness.
- Monitor results and review processes for continuous improvement.
专业提示
- Utilize advanced root cause analysis techniques such as Fishbone diagrams or the 5个为什么 to identify underlying issues comprehensively.
- Integrate CAPA processes with other quality management systems to ensure cross-functional collaboration and real-time feedback loops.
- Employ data analytics to predict potential non-conformances, allowing for proactive measures and increasing efficiency in preventive actions.
历史背景
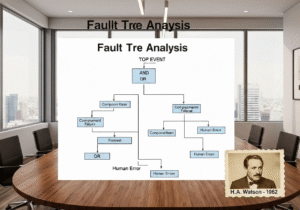
1962
1970
1972
1980
1980
1986
1986
1960
1963
1970
1980
1980
1980
1986
1987
(如果日期不详或不相关,例如 "流体力学",则对其显著出现的时间作了四舍五入的估计)。











相关文章
肌肉骨骼不适调查表
多变量测试(MVT)
多元回归分析
动作捕捉系统
MoSCoW 方法
情绪中值测试