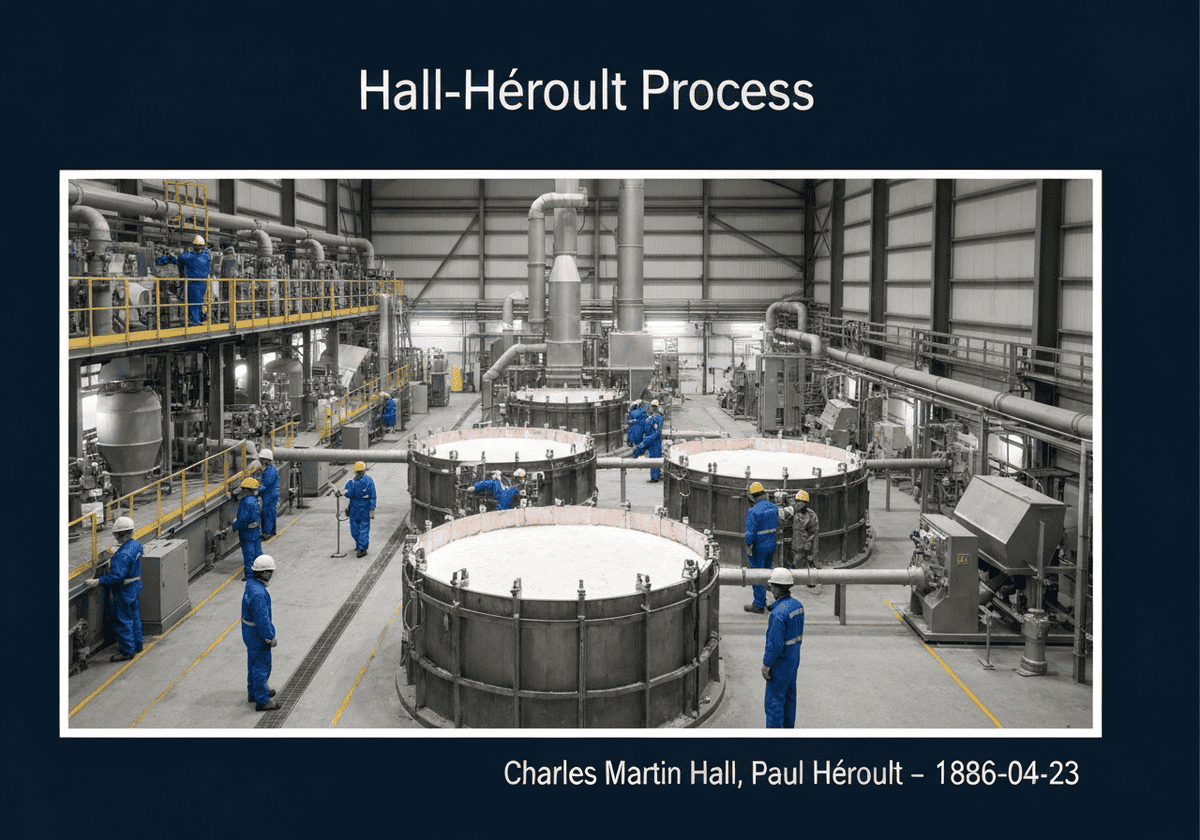
The Hall-Héroult process is the major industrial 方法 for smelting aluminum. It involves dissolving alumina (aluminum oxide, Al₂O₃) in molten cryolite (Na₃AlF₆) and electrolyzing the molten salt bath. Aluminum metal is deposited at the cathode, while oxygen from the alumina reacts with the carbon anode, producing carbon dioxide. This process made aluminum widely available and affordable.