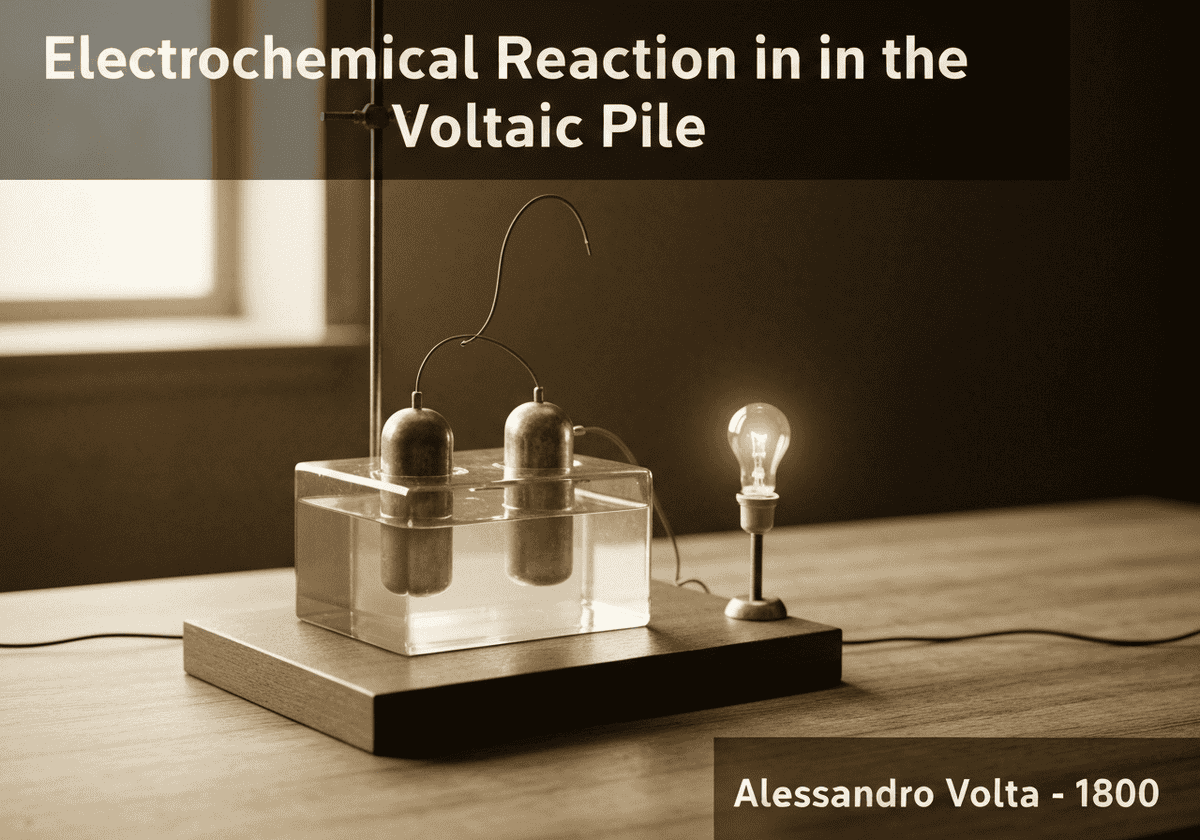
The electric current in a Voltaic pile is produced by a redox reaction. At the zinc anode, zinc metal is oxidized, releasing two electrons per atom ([latex]Zn \rightarrow Zn^{2+} + 2e^{-}[/latex]). These electrons travel through the external circuit to the copper cathode. There, hydrogen ions from the aqueous electrolyte are reduced, forming hydrogen gas ([latex]2H^{+} + 2e^{-} \rightarrow H_2[/latex]).